1. Background
Necrotizing enterocolitis (NEC) is a severe acquired disease of the digestive system in the neonatal period. It is defined as an acute intestinal syndrome, caused by various factors and mechanisms. This disease mainly affects premature and very-low-birth-weight infants (1). The mortality rate of NEC is as high as 20 - 30%, and 50% of children with NEC require surgical treatment (2). The high risk of neurological sequelae and gastrointestinal complications has been also reported among survivors (3). The early stage of NEC is mainly characterized by non-specific clinical manifestations. Conventional laboratory tests, such as routine blood collection, white blood cell count, procalcitonin (PCT), and C-reactive protein (CRP), lack specificity. Therefore, early diagnosis of NEC can be challenging.
The diagnosis of NEC can be clearly established when the disease has progressed into the middle and late stages (4). As the etiology and pathogenesis of NEC have not been studied comprehensively, the generally accepted mechanism is that the cascade of inflammatory reactions causes inflammatory damage to the intestinal wall, resulting in the occurrence and progression of NEC (5). The inflammatory response plays a key role in the pathogenesis of NEC. The CD64 and CD11b neutrophils can be found in the complement system. They significantly contribute to the body's defense response to microorganisms and immune regulation (6).
Although the NEC pathogenesis is not clear, some researchers believe that NEC and infection have a cause-effect relationship, as the production of large amounts of endotoxins by bacteria leads to the significant release of inflammatory mediators and results in inflammatory cascade reactions, intestinal wall erosion, and necrosis (7). The complement system plays an important role in the production of neutrophils, as CD64 and CD11b neutrophils can be extensively found in the complement system as cell surface molecules (8, 9). Therefore, the complement system is majorly involved in the progression of NEC. Moreover, the expression level of species in NEC is of great importance.
2. Objectives
Research on CD64 and CD11b neutrophils has mainly focused on neonatal infection and neonatal septicemia, and these indices have been confirmed as new indicators of neonatal inflammatory responses (10). However, CD64 and CD11b have been less studied in neonatal NEC. Therefore, this study aimed to analyze the expression of CD64 and CD11b in neonatal NEC and to further explore their diagnostic value for NEC to find better biological indicators.
3. Methods
3.1. Study Population
A total of 138 newborns, who were treated at the Department of Neonatology of Sanmenxia Central Hospital from October 2018 to march 2020, were selected as the study population. This study was approved by the ethics committee of our institution, and informed consent forms were signed by all guardians of the newborns.
The NEC group consisted of 69 NEC patients, who were admitted to the neonatal intensive care unit (NICU) and were diagnosed with NEC from October 2018 to march 2020. The diagnostic criteria were based on Bell’s NEC staging (11). The NEC group was divided into group I (n = 32 cases), group II (n = 23), and group III (n = 14), according to the staging criteria. The exclusion criteria were as follows: gestational age < 26 weeks and > 42 weeks; neonatal weight < 500 g and > 4200 g; primary or secondary immunodeficiency; congenital malformations of the digestive tract; severe liver or kidney disease; agranulocytosis; severe congenital heart disease; chromosomal abnormalities; and diseases that may lead to neutrophil deficiency during the perinatal period.
On the other hand, the control group consisted of 69 non-NEC newborns, who were admitted to the hospital during the same period as the NEC group. The non-NEC newborns were matched with the NEC group in terms of general information, including gestational age, sex, and weight; the two groups were matched at a 1:1 ratio. The exclusion criteria for the control group were as follows: gestational age < 26 weeks and > 42 weeks; neonatal weight < 500 g and > 4200 g; hypoxia; infection; asphyxia; systemic inflammatory response syndrome (SIRS); and genetic metabolic diseases. Also, the exclusion criteria for infection were positive blood culture, increased white blood cell count; and increased CRP and PCT.
3.2. CD64 And CD11b Quantification
The femoral vein blood (1 mL) was collected in an EDTA tube within two hours after diagnosis. The blood samples were collected when the circulatory system was stable to reduce the risks. All specimens were tested within four hours according to the following procedure. First, two test tubes were prepared. For this purpose, 20 uL of CD45-ECD, 20 uL of CD64FITC, and 20 uL of CD11b PE were added to the first test tube. Then, 20 uL of CD45-ECD, 20 uL of IgG1-FITC, and 20 uL of IgG1-PE were added to the second tube. Afterward, 50 uL of whole blood anticoagulant was added to each tube, gently mixed, and incubated for 10 - 15 minutes at room temperature. Following that, 500 uL of erythrocyte lysate was added to each tube. After mixing with a vortex mixer, the specimens were left at room temperature for 10 - 15 minutes.
Next, 2 mL of Phosphate buffer solution (PBS) was added to each tube and centrifuged for five minutes at 1200 rpm after vortex mixing. Then, the supernatant was discarded, and 0.5 mL of phosphate-buffered saline (PBS) was added to each tube. After vortex mixing, it was tested on a computer. A software program was used to collect the data related to the FITC-conjugated CD45 antibody for calibration, and 30,000 cells were obtained from each sample. The neutrophil, lymphocyte, and monocyte groups were delineated to analyze the mean fluorescence intensity (MFI) of CD11b and CD64. There was no reference for measuring the MFI of CD64 and CD11b neutrophil surfaces alone. Therefore, the CD64 and CD11b expression was expressed in exponential forms in this study.
3.3. CRP Measurements
For this purpose, the latex immunoturbidimetric assay (Meikang Biotech Co. Ltd., China) was used on Beckman AU5800 analyzer.
3.4. Statistical Analysis
Data were analyzed in SPSS version 22.0. The normal distribution of data was examined using Shapiro-Wilk test. Bartlett’s test was used to analyze the homogeneity of variance. Data with a normal distribution are presented as mean ± standard deviation (SD). For comparisons between two groups, independent samples t-test was performed, while for multi-group comparisons, one-way ANOVA was used. For data without a normal distribution or homogeneity of variance, the median and interquartile boundary values (p25, p75) were measured. Also, comparisons between two groups were performed by Wilcoxon signed-rank test, and multi-group comparisons were performed using Kruskal-Wallis test by ranks.
the quantitative data are expressed as percentage (%), and Chi-square test was used for evaluations. The False Discovery Rate (FDR) correcting was used for the results of pairwise comparison between Multiple groups. Moreover, the diagnostic value of CD64 and CD11b indices and CRP level was evaluated by the receiver operating characteristic curve (ROC) and the area under the curve (AUC). The CD64 and CD11b indices and their cut-off values for the best diagnostic efficacy were determined as the classification criteria by the Kolmogorov–Smirnov (K-S) test. The diagnostic value of the combination of CD64, CD11b, and CRP indices was evaluated by McNemar’s test and kappa statistic. All hypothesis tests were bilateral in this study, and differences were statistically significant at P < 0.05.
4. Results
4.1. Demographic Characteristics
This study included 69 NEC children as the NEC group. In this group, 35 (50.7%) subjects were male, and 34 (49.3%) were female. The mean birth weight of the NEC group was 1654 ± 373 g, the corrected gestational age was 32.29 ± 1.85 weeks, and age at sampling was 11.6 ± 3.11 days. Also, among 69 control subjects, 35 (50.7%) were male, and 34 (49.3%) were female. The average birth weight of this group was 1746 ± 400 g, the corrected gestational age was 33.24 ± 1.98 weeks, and the sampling age was 11.7 ± 3.14 days. Differences in sex, corrected gestational age, mean birth weight, and sampling age were not significant between the groups (P > 0.05), as shown in Table 1.
| Groups | N | Sex (%) | Birth Weight (g) | Corrected Gestational Age (Weeks) | Age at Sampling (Days) | |
|---|---|---|---|---|---|---|
| Male | Female | |||||
| Control group | 69 | 33 (47.8) | 36 (52.2) | 1746±400 | 33.24±1.98 | 11.7±3.14 |
| NEC group | 69 | 35 (50.7) | 34 (49.3) | 1654±373 | 32.29±1.85 | 11.6±3.11 |
| F-value | 0.114 | 0.199 | 0.544 | 0.11 | ||
| P-value | 0.736 | 0.787 | 0.7053 | 0.849 | ||
4.2. Comparison of CD64 and CD11b Indices and CRP Level Between the Groups
By comparing the CD64 and CD11b indices and CRP level between the groups, it was found that the level of these three markers in the NEC group was higher than the control group (Table 2). The CD64 and CD11b indices and CRP level were also compared between the groups with different stages of NEC. These indices were the highest in stage III, followed by stage II and stage I (Table 3).
| Groups | N | CD64 Index | CD11b Index | CRP |
|---|---|---|---|---|
| Control group | 69 | 0.76 (0.33, 2.00) | 0.95 (0.65, 1.56) | 2.13 (0.87, 5.26) |
| NEC group | 69 | 2.27 (0.86, 4.54) | 1.97 (0.66, 4.25) | 4.76 (1.79, 11.8) |
| Statistics | 17.5 | 10.2 | 9.85 | |
| P-value | 2.76e-05 | 1.42e-03 | 1.70e-03 |
| Groups | N | CD64 Index | CD11b Index | CRP Level |
|---|---|---|---|---|
| NEC I | 32 | 0.87 (0.62,1.70) | 0.91 (0.52,1.59) | 2.98 (1.18,5.8) |
| NEC II | 23 | 3.42 (2.30,4.68) | 3.20 (1.53,4.67) | 6.48 (2.1,11.3) |
| NEC III | 14 | 8.97 (6.17,10.9) | 8.02 (3.84,9.94) | 14.9 (4.31,18.7) |
| Statistics | 28.6 | 24.9 | 10.8 | |
| P-value | 6.22e-07 | 3.85e-06 | 4.48e-03 |
4.3. Performance Evaluation of CD64 and CD11b Indices and CRP Level
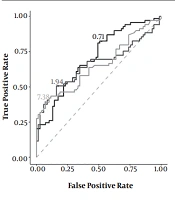
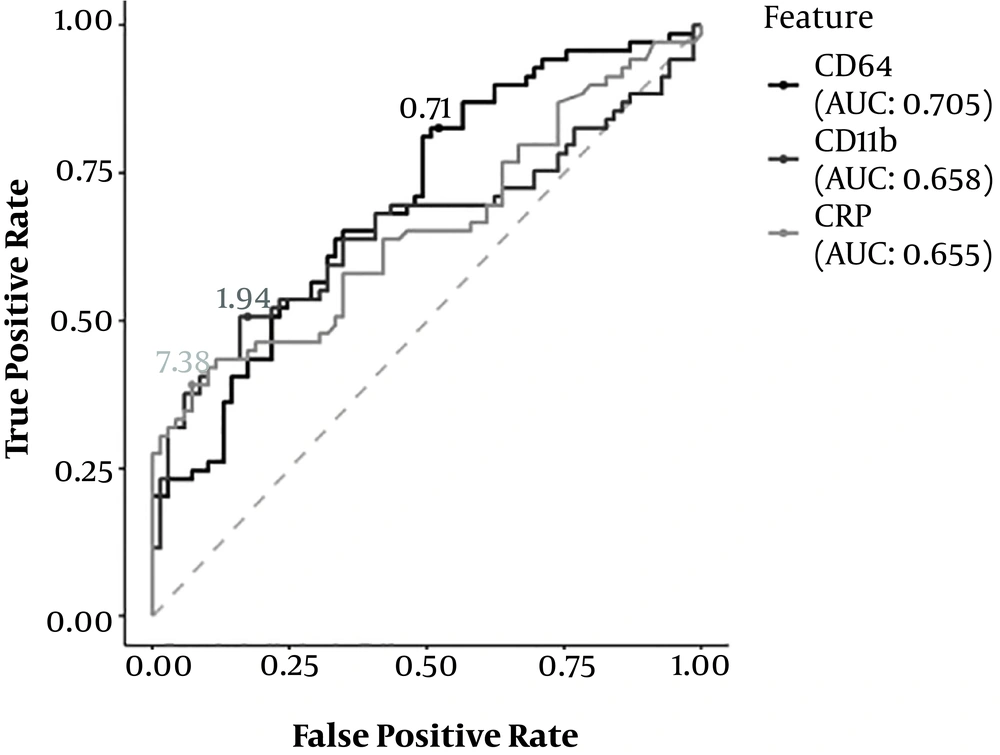
To evaluate the efficacy of CD64 and CD11b indices and CRP level as diagnostic markers of NEC, a ROC curve analysis was conducted, and the area under the curve (AUC) of the three indices was calculated. The AUC of the CD64 index was 0.707, the AUC of the CD11b index was 0.658, and the AUC of the CRP level was 0.655. All three indices showed a certain diagnostic efficacy. The diagnostic efficacy of the CD64 index was the highest, followed by the CD11b index and CRP level (Figure 1).
4.4. Estimation of CD64 and CD11b Indices and CRP Level
The K-S test was used to estimate the CD64 and CD11b indices and the cut-off value of CRP to find the index with the best diagnostic efficacy for NEC. The best cut-off value of CD64 was 0.71 (K-S value = 0.304), the best cut-off value of CD11b was 1.94 (K-S value = 0.333), and the best cut-off value of CRP was 7.38 (K-S value = 0.319). Therefore, a CD64 index ≥ 0.71, a CD11b index of 1.94, and a CRP level ≥ 7.38 were recognized as the best diagnostic criteria for NEC. Moreover, the sensitivity and specificity of CD64 and CD11b indices and CRP level, as well as their combination, were analyzed and compared. The CD64 index showed the highest sensitivity (82.7%), followed by CD11b (50.8%) and CRP (39.1%). On the other hand, the CRP level had the highest specificity (92.4%). The combined CD64 and C11b (more than one positive index) indices showed the highest sensitivity (89%), while the combined CD64 and C11b (two positive indices simultaneously) indices showed the highest specificity (87%) (Table 4).
| Methodology | Critical Threshold | AUC | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
| CD64 index | ≥ 0.71 | 0.707 | 82.7 | 48.0 |
| CD11b index | ≥ 1.94 | 0.658 | 50.8 | 82.6 |
| CRP level | ≥ 7.38 | 0.655 | 39.1 | 92.4 |
| Combined CD64 and C11b (more than one positive index) | 89 | 47.8 | ||
| Combined CD64 and C11b (two positive indices simultaneously) | 50.3 | 87.0 |
5. Discussion
Neonatal NEC occurs mainly in premature infants. If NEC occurs at a younger age, it appears later in life, and the fatality rate increases (12). Since NEC is associated with high morbidity and mortality, rapid progression, and poor long-term prognosis, it is a major challenge for premature newborns. In recent years, despite an increase in the survival rate of premature infants, the incidence of NEC continues to rise. Based on the clinical symptoms, laboratory indicators (e.g., routine blood collection, CRP level, white blood cell count, PCT, and other non-specific indicators) and imaging findings are used as the criteria for diagnosis and treatment of NEC. Early NEC is often misdiagnosed with the early manifestations of sepsis. Once typical imaging findings appear, NEC may progress into severe stages. Therefore, the best treatment time is missed, and the disease develops into severe NEC. Currently, the best surgical treatment for NEC is difficult to define, and preventing the disease is quite challenging (13). Accordingly, finding suitable biomarkers for NEC diagnosis has become one of the main research topics in recent years.
CD64, as one of the IgG receptors, is expressed in small amounts on the surface of neutrophils, resulting in infection or endotoxin invasion. The surface expression of CD64 is significantly upregulated within 4 - 6 hours of the inflammatory reaction (14). Many studies have shown that CD64, as a new biological marker, can be used as an effective supplementary tool to determine infection and non-infection. In this regard, Xiong Ray David et al (15) found that the CD64 index increased in the early stage of infection. Further research also showed that CD64, as a single index for the diagnosis of neonatal septicemia, has higher sensitivity and specificity than the single conventional CRP or PCT index (16). Lam HS et al (17, 18) indicated that CD64 can be used as a biological marker for routine monitoring of neonatal NEC and sepsis. Overall, various studies have shown that CD64 may be an excellent biological marker and that it can be used as a new biomarker of inflammatory response in future comprehensive studies.
CD11b is a glycoprotein molecule on the cell surface, which can specifically bind to the complement components or fragments of complement proteins and can be found in a low-expressed non-activated state on the surface of common mature neutrophils. The upregulation of CD11b is also considered a marker of early inflammation. In previous studies, it was found that the expression level of CD11b increased in confirmed cases of infection, but not in the non-infection group (19, 20). Further research indicated that the expression level of CD11b increased three days before the clinical presentation of sepsis and NEC, especially in very-low-birth-weight infants (21). Overall, the literature suggests that CD11b has predictive and diagnostic values in neonatal infectious diseases.
In the present study, the CD64 and CD11b indices and the CRP level of the NEC group were significantly higher than the control group. The CD64 and CD11b indices and CRP level are related to the occurrence of NEC. As NEC progresses, the CD64 and CD11b indices significantly increase; therefore, these two indices may be correlated with the degree of injury in NEC. On the other hand, the CD64 and CD11b indices significantly decreased during NEC recovery in each stage of NEC. The present results indicated a relationship between the CD64 and CD11b indices and the outcomes of the disease. Our results are consistent with the findings reported by Lam et al (17, 18).
By evaluating the diagnostic efficiency of CD64 and CD11b indices and CRP level, the CD64 index alone showed the highest sensitivity for diagnosis of NEC, while the sensitivity of CRP alone was the lowest. Although the CD11b index alone has high specificity, its sensitivity is not ideal. Since the development of NEC involves a pathophysiological process that combines immunity, infection, and energy metabolism, cytokine storms consume more energy in the early stages of the disease, resulting in insufficient energy supply and reduced expression of CD64 and CD11b in the later stages. The combination of these three indices may lead to the misdiagnosis or missed diagnosis of critical NEC in children. The combination of CD64 and CD11b indices showed the highest diagnostic sensitivity for NEC. Also, the results of this combined method for different NEC stages showed high sensitivity. Overall, the combination of CD64 and CD11b indices can be used in the differential diagnosis of NEC.
The normal expression of CD64 and CD11b in the neonatal peripheral blood and pathogenesis of NEC are not completely clear. Since the sample size included in this study was relatively small, the control group consisted of mostly non-healthy neonates, and daily dynamic monitoring was not possible (since multiple punctures increase pain and infection in children), the practical application of CD64 and CD11b indices needs to be further explored and combined with other routine laboratory markers and imaging examinations for further analysis.
In conclusion, the present results revealed that the expression of peripheral blood CD64 and CD11b significantly increased in neonatal NEC and were correlated with the severity of NEC; these findings are consistent with previous studies (17). The literature also suggests that the use of these two indices in the diagnosis of NEC has higher sensitivity than CRP and that the combination of these two indices can improve the diagnostic efficacy. This study provided new insights for the diagnosis and treatment of NEC and suggested practical markers for reducing the occurrence of serious NEC complications and improving the long-term prognosis of this disease.
5.1. Conclusion
Based on the findings, the expression of peripheral blood CD64 and CD11b increased in children with neonatal NEC, depending on the severity of the disease. Therefore, monitoring changes in these two indices has a high clinical value for the diagnosis of NEC. It can be concluded that the combined use of these two indices is more valuable in the diagnosis of neonatal NEC.