1. Background
Gastroesophageal reflux (GER), defined as physiologic passing of gastric content back to the esophagus with/without regurgitation or vomiting (1), which occurs in about two-thirds of healthy infants (2).
GER symptoms can happen in the first month of life, like recurrent regurgitation (spitting) and/or vomiting which is associated with transient relaxation of the lower esophageal sphincter regardless of swallowing (3, 4). When the bothersome symptoms like excessive crying, arching back, feeding refusal, irritability, and fussiness are reported, gastroesophageal reflux disease (GERD) happens that can lead to complications, such as esophagitis, failure to thrive, recurrent pneumonia and apnea (1, 5).
Differentiating between GER and GERD in the infant population is too unclear due to the difficulty of accurate definition of troublesome symptoms, and also the differentiation in the assessment of the parent/caregiver and even the physician about the severity of the symptoms (1, 6, 7). Generally, clinically diagnosis means, history taking and physical examination is the way of decision making about GER/GERD (8, 9).
Non-pharmacological treatment is the first step of infants’ treatment with GER and GERD, which leads to minimizing the symptoms (1, 10-13). Feeding and positioning changes are the main parts of the non-pharmacologic therapy of GERD (14). Increasing feeding frequency with reducing feeding time (volume) are the two main strategies of feeding-style correction which is mostly observed in breastfeeding versus formula-feeding (15). Studies have shown that the symptoms of GERD are lower in breastfed infants than in formula-fed infants (16). It is demonstrated that in breastfed infants, there are significantly shorter duration of GERD symptoms, lower esophageal pH, and more rapid gastric emptying than formula-fed infants (17-19). Hence, Breastfeeding is the best way of feeding in infants with GER and GERD due to these positive effects of breastfeeding on symptoms of GERD.
In breastfeeding babies, one of the feeding correction strategies to reduce regurgitation, is to modify the maternal diet, which usually means the exclusion of egg and cow’s protein from lactating mother diet for a 2 - 4 week trial in infants with cow’s milk allergy not GERD (12, 20, 21).
Iranian traditional medicine (ITM) is one of the complementary and alternative medicines (CAM) based on numerous practical evidence, and its history belongs to more than 2000 years ago (22). Avicenna (980 - 1037 AD) (23) a polymath and one of the most significant physicians believed like other Persian scholars, that the first step in treating of many infantile diseases is the treatment of her/his nursing mother through lifestyle modification, especially dietary modifications (24). The GERD symptoms were described under the title “Qay-el-Mobarrah” in pediatric medicine section of ITM references, which means “frequent vomiting” that is more prevalent in the infantile population (24-26).
2. Objectives
Accordingly, for the first time, we hypothesized that modification of maternal eating and drinking principles and diet based on ITM could be helpful for infantile GERD. Therefore, we designed a randomized clinical trial to evaluate the effect of maternal eating habits and diet on under 5 - month old infants with GERD symptoms.
3. Methods
3.1. Trial Design
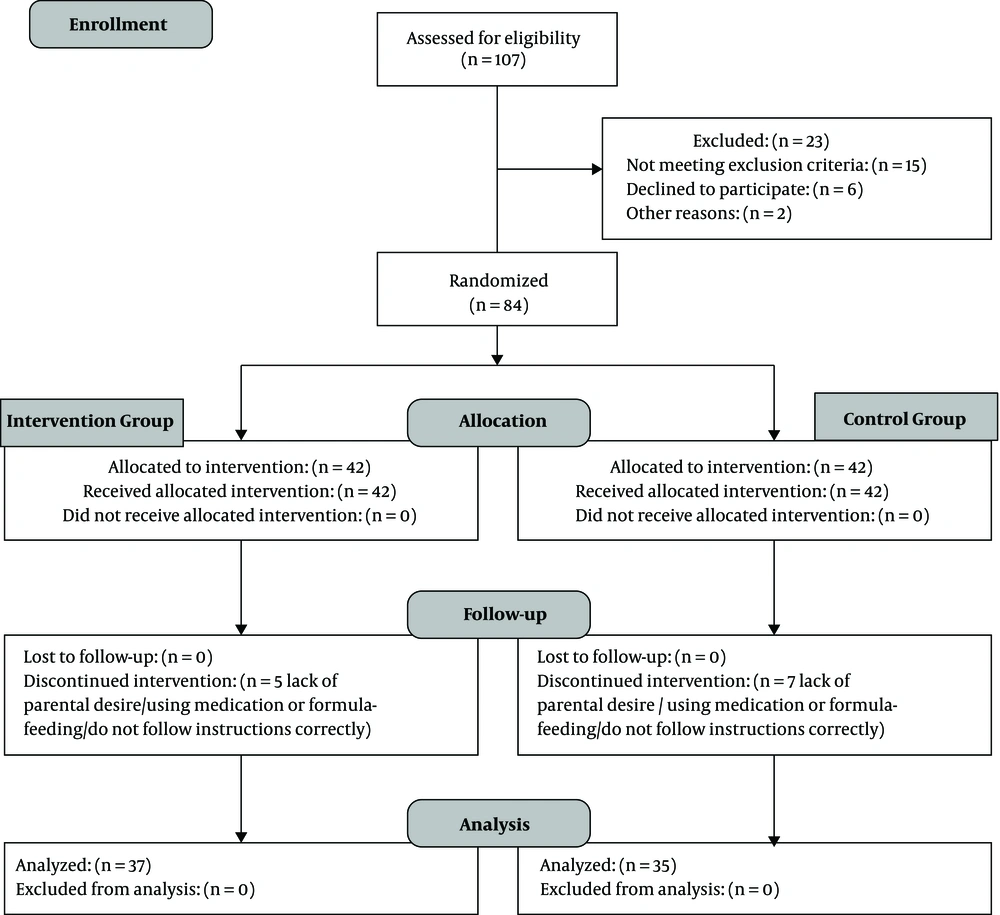
This is a two-arm parallel randomized, controlled clinical trial that compared the efficacy of “breastfeeding mother's dietary principles based on Iranian Traditional Medicine” with “lifestyle modification based on conventional medicine” on 0 - 5 months old exclusively breastfed infants, with clinical diagnosis GERD. There were not any changes in the study design after trial commencement. Reporting of this randomized controlled trial, is according to the latest version of CONSORT (Consolidated Standards of Reporting Trials) statement in 2010 (Figure 1) (27).
3.2. Participants
Infants with the clinical diagnosis of GERD, which were under 5 months old due to being in the exclusively breastfeeding course, were enrolled in this trial. Inclusion criteria were infants under 5 months old with mild to moderate infantile GERD, in whom the diagnosis was clinically confirmed by the pediatric gastroenterologist. Signs and symptoms that were the basis for diagnosing GERD in infants, through history and physical examination included recurrent regurgitation with/without vomiting, irritability, feeding refusal, arching back and persisting hiccups (1, 5).
Participants were excluded if there was a clinically significant disease in mother or infant, which led to medical or surgical therapy or prohibition of breastfeeding, and also other types of gastrointestinal diseases such as hiatal hernia or history of esophageal atresia in infants, due to their effect on the course of treatment and need for non-pharmacological treatment, such as drug therapy and/or surgery.
This study was enrolled in the traditional persian medicine clinic of Shahed University and Ali-Asghar Pediatrics Hospital of Iran University of Medical Sciences, both located in Tehran, Iran from 2018 Feb to 2020 Feb.
3.3. Interventions
After confirmation of the diagnosis of GERD by a pediatric gastroenterologist, at the first clinical visit (week 0) and after the randomization process, symptoms were evaluated through the Age-Specific Gastroesophageal Reflux Disease Questionnaire of Infants (GSQ-I) for all infants by the main researcher of the study. The frequency and usual severity of these symptoms were evaluated in the preceding 7 days: vomiting/regurgitation, irritability/fussiness, refusal to feed, choking/gagging, back arching, and episodes of hiccups. The parents were asked the number of times of each symptom (zero and more) and the score of usual severity of them on a scale from one (not too severe) to seven (very severe) (28).
The mothers of the intervention group were trained with dietary principles based on Persian Medicine. These instructions were developed in the form of an educational pamphlet: to eat one type of food at each meal and avoid the consumption of salads, yogurt or pickles with food, to eat slowly and chew food thoroughly, to avoid drinking water or other drinks from fifteen minutes before to one and a half hours after a meal (to compensate for the need to fluids between main meals), to avoid eating a meal or snacks when the stomach is full from the last meal (satiety), and a short list of cold and wet-natured foods to avoid them. Based on Iranian Traditional Medicine these principles affect the digestion process and improve the quality of breast milk, so can reduce the symptoms of Gastroesophageal reflux in infants (24, 29). In order to observe ethical considerations, lifestyle modification means correction of breastfeeding methods and positioning, and limitation of smoking were trained to mothers of both intervention and control group through a pamphle (1, 16). Our feeding advices included to decrease the amount of feeding in each time while increasing the frequency of breastfeeding, and post-breastfeeding burping, and ensure that both the nipple and a large area of areola were in the infant’s mouth (latching on). Also for positioning modifications, we recommended keeping infant in upright position for 20 minutes after each breastfeeding and to avoid prone and lateral position at sleeping time and when there is no supervision. The nursing mothers of the intervention and the control group were asked to apply these instructions for a 4-week course. All the teachings were done in person-to-person by the main researcher of the study.
The GSQ-I were asked at the end of the 4th week through the clinical visit and the mothers were told that the intervention course is over. Also, two weeks after the end of the intervention (week 6) GSQ-I was asked through phone contact to assess the persistence of response to treatment.
Infant weight and length were measured by the main researcher of the study, at the first clinical visit (week 0) and week 4 as the secondary outcome to indicate the rate of growth. A digital portable scale with an accuracy of 5 grams and a pediatric length mat were used to measure the weight and length of all infants.
3.4. Outcomes
The primary outcome measure was the changes in frequency and usual severity of symptoms in the previous 7 days in this trial, which was assessed individually and compositely. The Individual Symptom Score (ISS) was the result of multiplying the repetition number of each symptom in its severity score which is ranging from 1 (not at all severe) to 7 (most severe) in a recent week. The Composite Symptom Score (CSS) was the sum of ISSs. Mean of CSS in the first visit (week 0/baseline), end of the intervention (week 4), and 2 weeks after that (week 6). The changes of these scores (CSS0, CSS4, and CSS6) were the main outcome of this trial.
The secondary outcome measure was infant weight and length changes during the intervention course (week 0 and week 4).
3.5. Sample Size and Randomization
According to previous similar studies, with α = 0.05 and β = 90 %, the sample size was estimated to be 36 in each group and 72 overall. Eventually, seventy-two eligible infants and their nursing mothers were chosen purposefully and a code was allocated to each one of them. The participants were randomly divided into two groups, using block randomization with block size of two.
3.6. Statistical Methods
All data were analyzed using SPSS statistical software, version 24. For analyzing data, chi-square test, independent samples t-test and paired t-test were used. The significant value considered at P ≤ 0.05.
3.7. Safety Assessment
In order to assess the health status of the participants of the trial (nursing mothers and their babies), history taking and physical examination including measurement of infants’ weight and length were performed at baseline and week-4 by the major researcher who is a physician. Also, every two weeks a phone call contact with mothers was done and the major researcher’s phone number was given to the participants for probable question and problems of them.
4. Results
4.1. Participant Flow
From January 2018 to February 2020, 107 nursing mothers who had exclusive-breastfed infants under 5 months old with clinically diagnosed GERD were interviewed to assess the eligibility for the trial. At the beginning, twenty-three of them were excluded, so eighty-four of them were enrolled in this study and randomly allocated to intervention (n = 42) and control (n = 42) group. According to the inclusion criteria, all infants were healthy and did not get any medication except vitamin A and D supplement oral drop. Likewise, all mothers had no medical problem which is required in any therapeutic intervention. In the following, twelve participants (intervention group: 5, control group: 7) were excluded or refused to continue the study for some reasons such as failure to do instructions correctly and accurately, arbitrarily initiation of formula or medications (PPIs or H2 blockers) for babies or lack of parental desire. Eventually, seventy-two participants (intervention group: 37, control group: 35) completed the trial and were analyzed.
4.2. Baseline Data
There were no significant differences in demographic characteristics and baseline data, including infantile age at participation, gestational age at birth, gender, maternal age, level of education, and parents’ smoking status between intervention and control group, except the type of delivery (Table 1). All mothers were asked to record their diet for the last 72 hours, to prove that two groups were similar. Also, there were no significant differences in frequency and severity scores of GERD symptoms between two groups at baseline.
| Characteristics | Intervention Group | Control Group | P-Value |
|---|---|---|---|
| Infant age (month), mean ± SD | 1.62 ± 1.31 | 1.62 ± 1.37 | 0.562 |
| Infant weight (gram), mean ± SD | 5024.14 | 5074.86 | 0.757 |
| Infant length (centimeter), mean ± SD | 52.21 | 56.64 | 0.352 |
| Infant gender, No. (%) | 0.349 | ||
| Girl | 21 (56.8) | 16 (45.7) | |
| Boy | 16 (43.2) | 19 (54.3) | |
| Gestational age at birth, No. (%) | 0.297 | ||
| Preterm | 2 (5.4) | 1 (2.9) | |
| Term | 29 (78.4) | 32 (91.4) | |
| Post-term | 6 (16.2) | 2 (5.7) | |
| Delivery type, No. (%) | 0.023 | ||
| N.V.D. | 19 (51.4) | 27 (77.1) | |
| C.S. | 18 (48.6) | 8 (22.9) | |
| Mother age (year), mean ± SD | 29.08 ± 4.26 | 28.79 ± 5.09 | 0.536 |
| Mother education level, No. (%) | 0.716 | ||
| Associate’s degree and lower | 14 (37.8) | 14 (40.0) | |
| Bachelor’s degree | 15 (40.5) | 16 (45.7) | |
| Master’s degree and higher | 8 (21.6) | 5 (14.3) | |
| Smoking status of parents, No. (%) | 0.523 | ||
| Yes | 1 (2.7) | 2 (5.7) | |
| No | 36 (97.3) | 33 (94.3) |
Demographic Characteristics of Intervention and Control Group
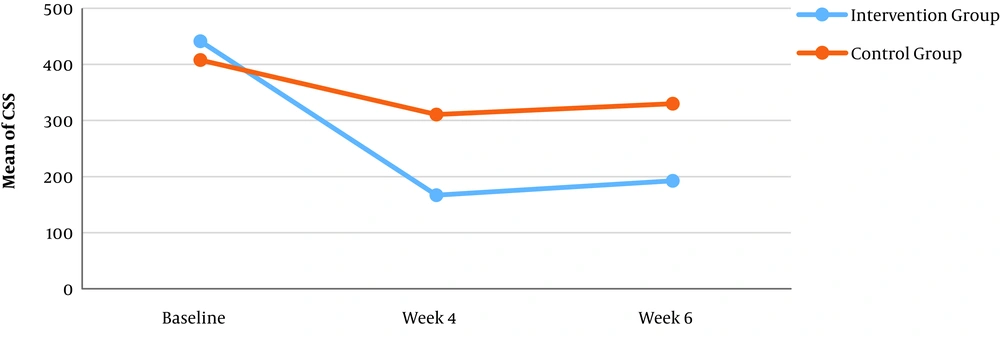
The main outcome of this trial was changes in the mean CSS values from baseline till the end of the intervention (week 4). The baseline mean CSS (mean CSS-0) values of two groups were similar (P = 0.531). The mean CSS values of week 4 (end of the intervention) was a significant reduction from the baseline in each group (P = 0.000), with statistical differences between the intervention and control group (P = 0.003). Also, the mean CSS-6 values showed a significant decrease compared to the baseline (P = 0.000) in both intervention and control groups (P = 0.019) (Table 2).
| Groups of Study | The Mean CSS Values ± SD | |||
|---|---|---|---|---|
| Week - 0 | Week - 4 | Week - 6 | P-Value | |
| Intervention | 441.45 ± 269.12 | 166.86 ± 127.95 | 192.43 ± 147.68 | 0.000 |
| Control | 407.71 ± 351.77 | 310.62 ± 238.70 | 330.02 ± 262.33 | 0.000 |
| P-value | 0.531 | 0.003 | 0.019 | |
Mean of CSSa at Baseline, Week - 4 and Week – 6
The mean decrease of CSS - 4 compared to CSS-0 in the intervention group was 275 with a confidence interval of 95% and was 97 in the control group, which indicates that the rate of reduction of frequency and severity of GERD symptoms in the intervention group was higher than that in the control group (Figure 2).
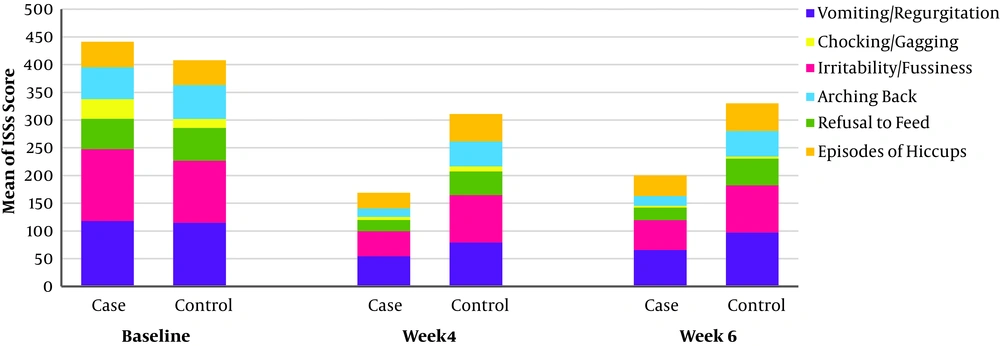
The frequency and usual severity of all symptoms in both treatment groups decreased significantly during the study (Figure 3).
At the baseline, the mean ISS values of all symptoms were similar except of chocking/gagging. The most common symptoms of the infants, who participated in this study, were Regurgitation/Vomiting. Episodes of Hiccups and Irritability/fussiness had the highest reduction among other symptoms (Table 3).
| Variables | Mean of ISS at Week - 0 | Mean of ISS at Week-4 | Mean of ISS at Week-6 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | P-Value | Intervention | Control | P-Value | Intervention | Control | P-Value | |
| Vomiting/Regurgitation | 117.81 | 114.91 | 0.908 | 54.35 | 79.23 | 0.080 | 65.73 | 97.00 | 0.108 |
| Irritability/Fussiness | 129.70 | 111.91 | 0.838 | 45.14 | 85.54 | 0.003 | 53.59 | 85.46 | 0.063 |
| Refusal to Feed | 55.03 | 59.14 | 0.657 | 20.49 | 42.47 | 0.008 | 22.95 | 47.94 | 0.028 |
| Chocking/Gagging | 35.16 | 15.83 | 0.004 | 5.30 | 8.80 | 0.146 | 3.08 | 3.80 | 0.653 |
| Arching back | 57.57 | 61.63 | 0.952 | 15.59 | 45.14 | 0.004 | 17.58 | 46.06 | 0.023 |
| Episodes of Hiccups | 46.19 | 44.89 | 0.457 | 27.89 | 49.37 | 0.000 | 27.59 | 49.77 | 0.005 |
Mean of the Individual Symptom Score (ISS) of Two Groups, at Week - 0 (Basement), Week - 4 (End of the Intervention), Week - 6 (Two Weeks After End of the Intervention)
The secondary outcome was infant weight and length changes during the trial as one of the growth parameters and efficacy of the treatment. At the end of the intervention (week - 4), the weight and length of all infants increased compared to baseline. The mean weight gain and length increase in the intervention group was higher than the control group without any significant differences between them (Table 4).
| Group | Mean of Weight Changes Between Week - 4 and Baseline (gr) ± SD | Mean of Length Changes Between Week - 4 and Baseline (cm) ± SD |
|---|---|---|
| Intervention | 842.97 ± 46.5 | 3.35 ± 0.18 |
| Control | 768.57 ± 82.46 | 3.21 ± 0.34 |
| P-value | 0.238 | 0.577 |
Assessment of Infant Weight and Length During the Study
5. Discussion
In this randomized controlled clinical trial, the effect of breastfeeding mother’s use of dietary instructions based on Iranian Traditional Medicine on infantile GERD was evaluated. According to the findings of this study, the mean ISSs and CSS values as the frequency and severity scales of symptoms of GERD were reduced in the intervention group more than the control group. In other words, although both ITM protocol and lifestyle modifications (feeding and positioning instructions) had a significant reduction in infantile GERD symptoms compared to baseline, it was demonstrated that ITM protocol is more effective in controlling infantile GERD than lifestyle modifications.
There were some studies about feeding modifications and positioning therapy for the treatment of infantile GER and uncomplicated GERD. The studies with the main concept of the feeding changes have evaluated the efficacy of more frequent feeding with reduced volume, applying feeding thickeners and prescription of protein hydrolysate formula on GERD symptoms. In reviewing articles on the type and methods of proper feeding for infants with GER/GERD, we are faced with the fact that exclusive breastfeeding can be a protective factor against reflux events. Heacock et al (1992) demonstrated that the duration of GER episodes was shorter in the breastfed neonates than the formula-fed neonates in their active sleep (18). Hegar et al (2009) through a prospective, observational study suggested that the exclusively breastfed infants regurgitated less than partially breastfed infants (30). In 2013 in Indonesia, Hegar et al again, showed that exclusively breastfed infants had 5 to 10 times lower frequencies of regurgitation/vomiting than formula-fed infants. Chen et al (2015) demonstrated that direct feeding from mothers’ breasts (not pumping) protected infants against reflux episodes, also any feeding method in combination with formula was a risk for gastroesophageal reflux (31).
In addition to the effect of feeding modalities (lower volume, more frequency), the composition of milk influences GER/GERD symptoms. Vandenplas et al (1998) showed that a low fat and high glucose polymer content of formula could be appropriate for regurgitation due to shorter gastric emptying duration (32). The biochemical composition of breast milk affect gastric emptying time in preterm infants, such as higher casein level (with a milk-fortifier) is equal to faster gastric emptying (33, 34). Based on these results we understand that, the type of milk composition can also affect the signs and symptoms of infantile GERD.
To date, based on our findings, the only intervention on nursing mother’s diet that affects regurgitation and/or vomiting in infants is the elimination of food sources of cow’s milk protein from the breastfeeding mother’s diet which is effective just in infants with a diagnosis of cow’s milk protein allergy (1, 5, 16). We found no more studies which evaluated the effect of other modifications of nursing mothers’ diet or habits on infantile GERD.
Recent studies show that maternal lifestyle, especially nutrition and physical activity, during pregnancy, postpartum, and breastfeeding period has the significant effects on infant health (35-39). Thus, the study about how to address this relationship could be one of the future plans for research on maternal and infant health. According to Persian Medicine pioneers believed maternal lifestyle, health level, and probable disorder could affect the quality and quantity of breast milk and accordingly affect the baby's health. In the same way, the correction of breast milk by caring for nursing mothers is the priority in the treatment plan for infant diseases (24-26, 40).
5.1. Conclusion
This is the first clinical trial to signify the effect of the nursing mother’s eating and drinking habits and diet on infantile gastroesophageal reflux. It is demonstrated that breastfeeding mothers’ eating and drinking principles and diet based on traditional persian medicine reduces the frequency and severity indexes of infantile GERD symptoms significantly. As this investigation showed the effect of one of the elements of the maternal lifestyle on the decrease of infantile GERD symptoms, in the future, further studies are needed to evaluate the direct effect of other maternal lifestyle principles on breastfeeding infants’ health, some illnesses, and treatment plan. Also, biochemical investigations are required to show changes in human milk composition by modification of maternal lifestyle.
5.2. Limitations
One of the major problems with this study was the discontinuation of breastfeeding, often as a result of misconceptions of the family or the wrong training of health workers. Another problem was the arbitrary prescribing of chemical and/or herbal medicines to infants by family members.