1. Background
Vulvovaginitis is a common problem in prepubertal girls, which is mainly the result of poor hygiene or nonspecific irritants as well as bacterial infection (1). Streptococcus pyogenes and Haemophilus influenzae (H. influenzae) are reported as the most common bacterial cause of vulvovaginitis in prepubertal girls (1-4). In 1987, MacFarlane first highlighted the relationship between H. influenzae and prepubertal vulvovaginitis (5). Cox’s (4) study suggested that H. influenzae was an underrated cause of vulvovaginitis among young girls. However, few studies have comprehensively explored the prevalence of H. influenzae vulvovaginitis in prepubertal girls. Furthermore, few studies with large sample sizes have been conducted in this regard.
2. Objectives
Accordingly, we described a four-year study from a tertiary university children’s hospital to determine the prevalence of H. influenzae vulvovaginitis in prepubertal girls and detect the antimicrobial resistance of H. influenzae strains isolated from vulval specimens.
3. Methods
3.1. Strain Collection and Identification
This retrospective analysis examined the data collected from prepubertal girls referred to the outpatient clinic of pediatric and adolescent gynecology at The Children’s Hospital, Zhejiang University School of Medicine, from January 2016 to December 2019. One vulval swab was taken for each for microscopic examination. Moreover, another vulval swab was received for culture. To this end, Columbia blood agar, Chocolate agar (i.e., Haemophilus and Gonorrhoeae selective chocolate agar), and Sabouraud’s agar were used. The suspected pathogens on Haemophilus selective chocolate agar were identified using the Matrix-Assisted Laser Desorption Ionization Time of Flight Mass Spectrometry (MALDI-TOF MS, Bruker).
3.2. β-Lactamase Detection and Antimicrobial Susceptibility Test
The antimicrobial susceptibility test was performed using disk diffusion, and the results were interpreted according to the Clinical Laboratory Standards Institute’s (CLSI) guidelines M100-S29. The resistance rates of H. influenzae isolates to ampicillin, amoxycillin-clavulanic acid, ampicillin-sulbactam, cefuroxime, ceftriaxone, cefotaxime, meropenem, levofloxacin, sulfamethoxazole-trimethoprim, azithromycin, and chloramphenicol (Oxoid, UK) were also detected. In this regard, H. influenzae ATCC49247 was used as a quality control strain, β-lactamase was detected by Cefinase disc (BioMérieux, France), and H. influenzae isolates resistant to three or more different types of antibiotics were defined as Multi-Drug Resistance (MDR) isolates.
3.3. Statistical Analysis
The antibiotic-resistant rates were analyzed with WHONET software version 5.6. The antibiotic-resistant rates between different groups were also compared and analyzed using the chi-square test. Medians (IQR) were used to describe age data, and P < 0.05 was considered as the significance level.
4. Results
4.1. Isolates and Distribution
From January 2016 to December 2019, 4142 vulval swabs were received from prepubertal girls with vulvovaginitis, and 649 swabs (15.7%) were obtained from 642 patients with H. influenzae. The number of specimens received was increased from 803 in 2016, 931 in 2017, 920 in 2018 to 1488 in 2019; however, the proportion of H. influenzae positives was decreased from 18.6% (149/803) in 2016, 15.5% (144/931) in 2017, 16.8% (155/920) in 2018, to 13.0% (194/1488) in 2019. The peaks were noticed from April to July in the vulval isolates positive for isolates of H. influenzae. The age of the children with the H. influenzae isolates ranged from 0.5 to 13 years; however, 477 persons (75%) were in the age range of 3 - 7 years, with a median of 5 years (IQR: 3).
4.2. β-Lactamase Detection and Antimicrobial Susceptibility Test
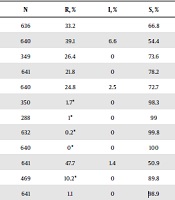
In this study, the ampicillin resistance rate was 39.1% (250/640), of which 33.2% of the strains (211/636) were for β-lactamase-positive isolates, and 6.6% of the strains (42/635) were β-lactamase-negative ampicillin-resistant (BLNAR) isolates. The resistance rates of H. influenzae isolates to amoxycillin-clavulanic acid, ampicillin-sulbactam, cefuroxime, ceftriaxone, cefotaxime, meropenem, levofloxacin, sulfamethoxazole-trimethoprim, azithromycin, and chloramphenicol were 26.4%, 21.8%, 24.8%, 1.7%, 1.0%, 0.2%, 0%, 47.7%, 10.2%, and 1.1%, respectively (Table 1). The resistance rates of H. influenzae strains to cefuroxime and azithromycin revealed significant statistical differences over different years (P < 0.05; Table 2). β-lactamase-positive H. influenzae strains showed significantly higher resistance to ampicillin, amoxycillin-clavulanic acid, cefuroxime, sulfamethoxazole-trimethoprim, azithromycin, and chloramphenicol, compared to β-lactamase-negative strains (P < 0.01; Table 3). BLNAR H. influenzae strains were all resistant to amoxycillin-clavulanic acid, ampicillin-sulbactam, and cefuroxime; however, they were susceptible to levofloxacin, azithromycin, and chloramphenicol (Table 4).
| Antibiotic | N | R, % | I, % | S, % |
|---|---|---|---|---|
| β-lactamase | 636 | 33.2 | 66.8 | |
| Ampicillin | 640 | 39.1 | 6.6 | 54.4 |
| Amoxycillin-clavulanic acid | 349 | 26.4 | 0 | 73.6 |
| Ampicillin-sulbactam | 641 | 21.8 | 0 | 78.2 |
| Cefuroxime | 640 | 24.8 | 2.5 | 72.7 |
| Ceftriaxone | 350 | 1.7* | 0 | 98.3 |
| Cefotaxime | 288 | 1* | 0 | 99 |
| Meropenem | 632 | 0.2* | 0 | 99.8 |
| Levofloxacin | 640 | 0* | 0 | 100 |
| Sulfamethoxazole-trimethoprim | 641 | 47.7 | 1.4 | 50.9 |
| Azithromycin | 469 | 10.2* | 0 | 89.8 |
| Chloramphenicol | 641 | 1.1 | 0 | 98.9 |
Antibiotic Resistances of H. influenzae Strains Isolated from Vulval Specimens, 2016 – 2019a
| Antibiotic | 2016 | 2017 | 2018 | 2019 | P-Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| N | R, % | N | R, % | N | R, % | N | R, % | ||
| β-lactamase | 148 | 31.8 | 142 | 26.8 | 156 | 35.9 | 192 | 37 | 0.209 |
| Ampicillin | 148 | 33.1 | 145 | 37.2 | 155 | 41.9 | 194 | 42.8 | 0.254 |
| Amoxycillin-clavulanic acid | - | - | - | - | 154 | 23.4 | 194 | 28.9 | 0.249 |
| Ampicillin-sulbactam | 149 | 16.1 | 145 | 26.9 | 155 | 21.3 | 194 | 22.7 | 0.161 |
| Cefuroxime | 149 | 17.4 | 145 | 26.2 | 154 | 23.4 | 194 | 30.4 | 0.048 |
| Ceftriaxone | - | - | - | - | 155 | 1.3* | 194 | 2.1* | 0.582 |
| Cefotaxime | 146 | 1.4* | 143 | 0.7* | - | - | - | - | 0.574 |
| Meropenem | 148 | 0* | 137 | 0.7* | 155 | 0* | 194 | 0* | 0.304 |
| Levofloxacin | 148 | 0* | 145 | 0* | 155 | 0* | 194 | 0* | - |
| Sulfamethoxazole-trimethoprim | 149 | 49 | 145 | 45.5 | 155 | 47.7 | 194 | 48.5 | 0.936 |
| Azithromycin | - | - | - | - | 155 | 7.1* | 191 | 14.7* | 0.027 |
| Chloramphenicol | 149 | 1.3 | 145 | 1.4 | 155 | 0 | 194 | 1.5 | 0.515 |
Antibiotic Resistances of H. influenzae Strains Isolated from Vulval Specimens Over Different Yearsa
| Antibiotic | β-Lactamase (+) | β-Lactamase (-) | P-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | R, % | I, % | S, % | N | R, % | I, % | S, % | ||
| Ampicillin | 211 | 98.6 | 0.5 | 0.9 | 424 | 9.9 | 9.4 | 80.7 | 0.000 |
| Amoxycillin-clavulanic acid | 126 | 38.9 | 0 | 61.1 | 222 | 18.9 | 0 | 81.1 | 0.000 |
| Ampicillin-sulbactam | 212 | 26.9 | 0 | 73.1 | 424 | 19.6 | 0 | 80.4 | 0.046 |
| Cefuroxime | 212 | 33 | 6.6 | 60.4 | 423 | 21 | 0.5 | 78.5 | 0.001 |
| Ceftriaxone | 127 | 3.1* | 0 | 96.9 | 222 | 0.9* | 0 | 99.1 | 0.272 |
| Cefotaxime | 83 | 1.2* | 0 | 98.8 | 201 | 1* | 0 | 99 | 0.626 |
| Meropenem | 210 | 0* | 0 | 100 | 417 | 0.2* | 0 | 99.8 | 0.608 |
| Levofloxacin | 211 | 0* | 0 | 100 | 424 | 0* | 0 | 100 | - |
| Sulfamethoxazole-trimethoprim | 212 | 59.4 | 0.5 | 40.1 | 424 | 42 | 1.9 | 56.1 | 0.000 |
| Azithromycin | 161 | 28* | 0 | 72 | 307 | 1* | 0 | 99 | 0.000 |
| Chloramphenicol | 212 | 3.3 | 0 | 96.7 | 424 | 0 | 0 | 100 | 0.001 |
Comparng Antibiotic Resistance Between β-Lactamase-Positive and β-Lactamase-Negative H. influenzae Strains Isolated from Vulval Specimens, 2016 - 2019a
| Antibiotics | N | R, % | I, % | S, % |
|---|---|---|---|---|
| Amoxycillin-clavulanic acid | 22 | 100 | 0 | 0 |
| Ampicillin-sulbactam | 42 | 100 | 0 | 0 |
| Cefuroxime | 42 | 100 | 0 | 0 |
| Ceftriaxone | 22 | 9.1* | 0 | 90.9 |
| Cefotaxime | 20 | 10* | 0 | 90 |
| Meropenem | 36 | 2.8* | 0 | 97.2 |
| Levofloxacin | 42 | 0* | 0 | 100 |
| Sulfamethoxazole-trimethoprim | 42 | 47.6 | 9.5 | 42.9 |
| Azithromycin | 24 | 0* | 0 | 100 |
| Chloramphenicol | 42 | 0 | 0 | 100 |
Antibiotic Resistances of BLNAR H. influenzae Strains Isolated from Vulval Specimens, 2016 - 2019a
4.3. MDR Pattern
Of the 642 H. influenzae isolates, MDR was present in 41 cases (6.4%). Ampicillin-sulfamethoxazole-trimethoprim-azithromycin resistance was the most prevalent resistance phenotype, which was detected in 16 isolates, representing 39% of the MDR strains (Table 5).
| MDR pattern | No. (%) |
|---|---|
| β-lactams-SXT-AZM | |
| AMP-SXT-AZM | 16 (39.0) |
| AMP-CXM-SXT-AZM | 4 (9.8) |
| AMP-CXM-AMC-SXT-AZM | 3 (7.3) |
| AMP-CXM-SAM-AMC-SXT-AZM | 3 (7.3) |
| AMP-AMC-SXT-AZM | 2 (4.9) |
| AMC-SXT-AZM | 1 (2.4) |
| AMP-CXM-SAM-SXT-AZM | 1 (2.4) |
| SAM-SXT-AZM | 1 (2.4) |
| CXM-SXT-AZM | 1 (2.4) |
| AMP-SAM-AMC-SXT-AZM | 1 (2.4) |
| AMP-CXM-SAM-AMC-CRO-SXT-AZM | 1 (2.4) |
| β-lactams-SXT-CHL | |
| AMP-SXT-CHL | 3 (7.3) |
| AMP-AMC-SXT-CHL | 1 (2.4) |
| AMP-SAM-SXT-CHL | 1 (2.4) |
| AMP-CXM-SXT-CHL | 1 (2.4) |
| β-lactams-SXT-AZM-CHL | |
| AMP-SXT-AZM-CHL | 1 (2.4) |
Main MDR Patterns of H. influenzae Strains Isolated from Vulval Specimens, 2016 - 2019
5. Discussion
Vulvovaginitis in prepubertal children is a common infection in clinical practice. Given the anatomy of the vulva at prepubertal age, it is vulnerable to infection in prepubertal children (1). Few hospitals can provide pediatric and gynecological outpatient services; hence, children with vulvovaginitis mainly receive the primary care (3). In this regard, few studies have comprehensively explored the prevalence of H. influenzae vulvovaginitis in prepubertal girls. To this end, the present study was performed at a tertiary university hospital providing specialist pediatric, and gynecological outpatient services.
Vulvovaginitis is one of the most common gynecological problems among prepubertal girls. In this regard, a multicenter study showed the leading cause of pediatric inflammatory vulvovaginitis to be the upper respiratory tract pathogens (6). A case report first documented the nose-hand-vagina method of transmission for vulvovaginitis (7), which assumed that respiratory pathogens were transmitted to the vulvar area via the hands (8). Accordingly, hand hygiene and behaviors would be an essential strategy to prevent vulvovaginitis in prepubertal girls.
Several studies have indicated that vulvovaginitis in prepubertal girls is mainly caused by the bacteria from the upper respiratory tract, S. pyogenes, and H. influenzae (1). H. influenzae more commonly caused vulvovaginitis than β haemolytic streptococci in Liverpool (9). However, H. influenzae is fastidious for growth requirements; hence, laboratories should not isolate it unless they cover the appropriate culture medium of Haemophilus for vulval swabs (10). In the present study, all the specimens were inoculated on the selective chocolate agar of Haemophilus to isolate H. influenzae.
Previous studies have described a variety of bacteria as the possible causes of vulvovaginitis in children. However, signs of inflammation associated with pure or predominant growth may be diagnostic relevance of pathogenic microorganisms (1). In this study, the large number of polymorphonuclear leukocytes in the microscopic examination revealed the inflammatory reaction, implying that the H. influenzae isolated from the vulval swabs was a possible pathogenic microorganism. In this study, of 4142 vulval swabs, 649 swabs (15.7%) were from children with H. influenzae. This issue was in agreement with the opinions described above, suggesting that H. influenzae was a common pathogen of vulvovaginitis in children in Zhejiang, China. The peaks of isolates were noticed during April-July in the vulval isolates, which was consistent with the peaks of respiratory tract specimens, suggesting that vulval H. influenzae strains might be transmitted from the respiratory tract (6, 11). The age of children with H. influenzae ranged from 0.5 to 13 years; however, 477 children (75%) were aged between 3-7 years. This finding was in line with those of the previous studies (11, 12).
Since the 1970s, ampicillin was used as an option to treat H. influenzae infections (13). In recent years, because of the extensive use of antibiotics, the drug resistance of H. influenzae to ampicillin has gradually increased. The ampicillin resistance rate of H. influenzae strains in China was increased from 12% during 2000 - 2002 (14) to 58.1% in 2016 (15). In this study, the ampicillin resistance rate was 39.1%, which was higher than the rate in genital strains (26.4%) and lower than that in respiratory strains (58.4%) in 2015, as reported by our research team (15). The ampicillin resistance of H. influenzae strains isolated from vulval specimens gradually increased from 33.1% in 2016 to 42.8% in 2018; hence, the ampicillin resistance of H. influenzae should be considered in clinical management. In the present study, the main mechanism of ampicillin resistance in H. influenzae isolates was the production of β-lactamase. This finding was in agreement with those in some other studies (12, 16, 17) and in contrast with those reported in Japan. Regarding the inconsistency of the findings, BLNAR accounted for more than 50% of cases after 2014 (18) and only 6.6% in this study, suggesting significant differences among different countries regarding the antibiotic resistance and mechanisms of H. influenzae isolates. Some studies have also compared H. influenzae resistance profiles between the respiratory tract and urinary tract (19), respiratory isolates and vaginal isolates (11), suggesting that the resistance profiles of H. influenzae vary greatly depending on the infection site. This finding also indicates that the optimal antibiotic treatment for H. influenzae may differ depending on the infection region and infection site. The resistance rates of the H. influenzae isolates to amoxycillin-clavulanic acid, and ampicillin-sulbactam were 26.4%, 21.8% in this study, which might be attributed to the BLNAR strains and the β-lactamase-producing clavulanic acid/amoxicillin-resistant (BLPACR) strains of H. influenzae. Furthermore, β-lactamase and PBP amino acid substitutions might be the mechanisms of BLPACR strains (20).
Generally, H. influenzae strains are highly susceptible to third-generation cephalosporins. The non-susceptibility rate of H. influenzae to third-generation cephalosporins was < 2% in the present study. This rate was much smaller than the rate reported in Iran (33.1%) (21) and Japan (49.4%) (22); however, it was similar to the rate of genital strains (5.5%) in China in 2015. In this regard, different infection sites may explain such an inconsistency. Typically, H. influenzae is sensitive to carbapenem; however, carbapenem-non-susceptible H. influenzae has also been reported in the literature (23). The present findings reported one H. influenzae strain non-susceptible to meropenem, whose mechanism is worthy of research in future studies.
This study showed the high prevalence of sulfamethoxazole-trimethoprim resistance (47.7%) among H. influenzae isolates; however, no significant difference was noticed between the present findings (47.7%, 306/641) in 2016 - 2019 and the previous ones (51.8%, 57/110) in 2015 (11). This might have been caused by the fewer applications of sulfamethoxazole-trimethoprim. Moreover, 10.2% of the H. influenzae isolates were resistant to azithromycin in this study. A significantly increased resistance was noticed during 2018 - 2019, which might be caused by the extensive use of azithromycin in respiratory infections in China. Furthermore, in the study, H. influenzae strains were all sensitive to levofloxacin, and 1.1% of H. influenzae strains were resistant to chloramphenicol. This is probably because these antibiotics are rarely used in children in China. MDR was observed in 41 cases (6.4%) of the 642 H. influenzae isolates. In line with the findings of the previous studies, the most prevalent resistance phenotype was ampicillin-sulfamethoxazole-trimethoprim-azithromycin resistance (15).
6. Conclusions
To the best of our knowledge, the present study represents the largest population-based study on H. influenzae vulvovaginitis among prepubertal girls in China. H. influenzae is considered as a common bacterial cause of vulvovaginitis in children in Zhejiang, China; hence, laboratories are recommended to routinely cover Haemophilus culture media for vulval specimens and consider the ampicillin resistance of H. influenzae in clinical management. A prominent strategy to prevent vulvovaginitis in prepubertal girls is to provide suggestions on hand hygiene and behaviors.