1. Background
Cardiac catheterization is a useful method for determining the anatomy and pressure in cardiac vessels and chambers (1-3). Contrast media injection would help in the better illustration of the anatomical details. Also, the usage of indicator substance injection would help find vascular branches. Further, blood sampling determines the oxygen saturation for finding the possible shunts (4). A stable cardiopulmonary status is essential to find the shunts, as well as oxygen and carbon dioxide pressure, before and during measurements (1, 3).
Anesthesia management for pediatric cardiac catheterization is a matter of debate (5-7). The shunts may be seen at different levels, and patients may be cyanotic (6). Further, young patients may not have the required cooperation, and the parents may not be able to offer assistance due to severe stress (6, 7). The diagnosis of cardiac anomalies is usually made by echocardiography, but the determination of therapeutic course and approaches is done through cardiac catheterization (8-10). Sedation and analgesia are usually useful with no intolerance in adult patients under cardiac catheterization (9, 10). However, in children and neonates, intravenous sedatives are not easily used, and general anesthesia is the main approach that may be also done by adding midazolam (11, 12). The maintenance of physiological and respiratory dynamic status during diagnostic cardiac angiography is crucial. General anesthesia is usually used for pediatric cases, and the recognition of the best method with minimal respiratory and hemodynamic effects is valuable (10-13). The use of general anesthesia in children may affect the hemodynamic parameters and diagnostic angiography results, thus necessitating the knowledge of such effects (4). Decreased apnea threshold and some degrees of respiratory depression during general anesthesia would lead to increased carbon dioxide pressure, as well as lung vascular resistance and pulmonary hypertension (4-6). Hence, the use of anesthesia methods with minimal hemodynamic and respiratory effects can increase diagnostic accuracy (14-16).
Since there are a few exclusive pediatric angiography centers, scarce studies have been done in this area. Regarding the fact that a significant change in the level of PCO2 could adversely affect angiographic measurements, we hypothesized that there would be no difference in PCO2 measurements between the groups (H0).
2. Objectives
In this study, the effects of Intermittent positive pressure ventilation (IPPV) versus Spontaneous Ventilation were determined on cardiorespiratory parameters in pediatric patients aged less than one year old undergoing angiography with general anesthesia.
3. Methods
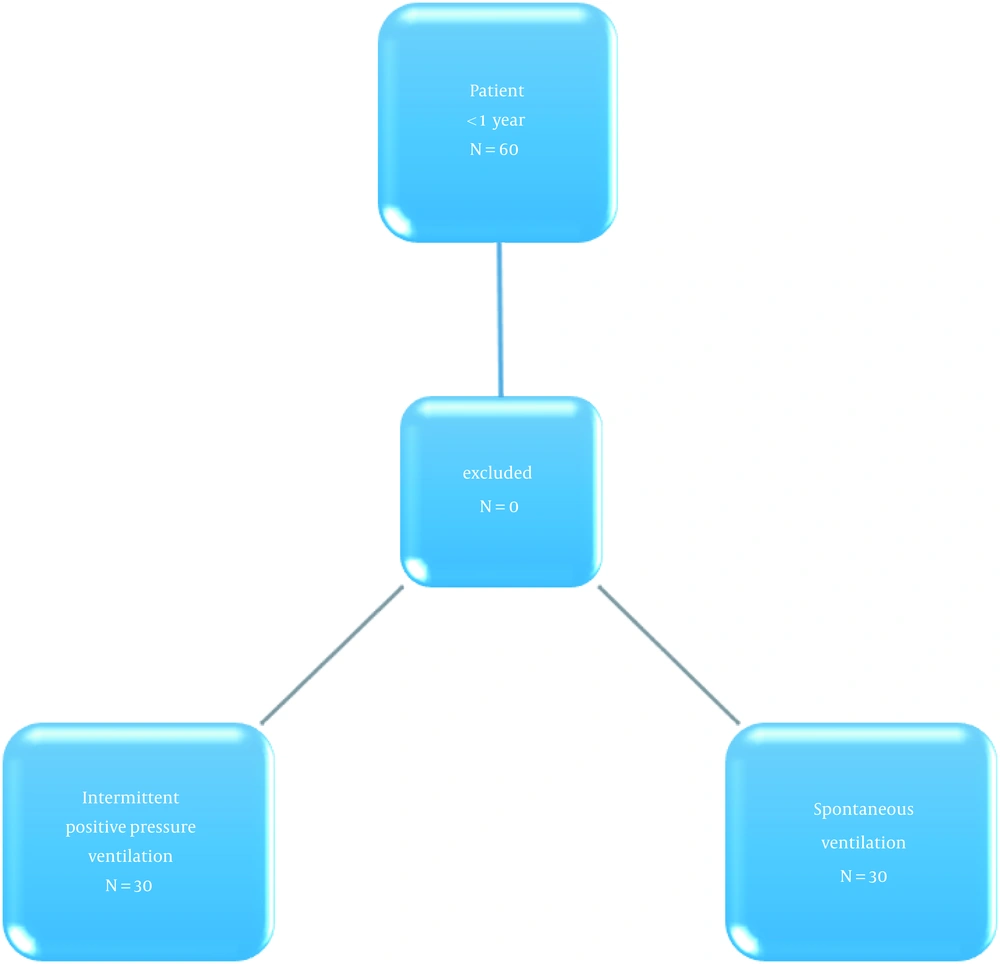
This double-blind randomized controlled trial was conducted on 60 children under one year old, referred to a Children’s Medical Center, Tehran, Iran. Informed consent was attained from the parents of the patients if the patients did not meet the following exclusion criteria: Pulmonary, renal, hepatic, and metabolic background diseases, previous cardiac and thoracic surgery, requiring over two ketamine doses, and receiving sedative or anti-convulsant therapeutics.
All patients had been admitted, and the Emla cream 5% (EMLA AstraZeneca) had been used in the inguinal region 15 minutes before the procedure. Anesthesia induction was performed after the patients were entered the catheterization laboratory with their mothers while being monitored by ECG and pulse oximetry, besides non-invasive blood pressure. Then, the patients were equally allocated into the two groups by simple random sampling (Figure 1). In group A, anesthesia induction was done by intravenous injection of ketamine 1 mg/kg and mask oxygenation plus Bain Circuit with oxygen 100% and sevoflurane 1.5 Minimum Alveolar Concentration (MAC) with spontaneous ventilation maintenance. Tracheal intubation was performed after one minute. After approval of the true position of the tracheal tube and spontaneous ventilation, the tracheal tube was fixed, and sevoflurane 1.5 MAC and oxygen 21% were administered. After 10 minutes and following preparation and drape and pushing the angiography needle to the skin, the sevoflurane dose was decreased to 1 MAC. Intravenous ketamine 0.5 mg/kg was used once repeat needling was needed or the patient moved. In group B, with the same induction method, ketamine 1 mg/kg was injected intravenously plus mask oxygenation and Bain Circuit with oxygen 100% and atracurium 0.5 mg/kg for maintenance. After tracheal intubation and confirming the true location, the anesthesia continued with sevoflurane 1.5 MAC and oxygen 21%. After 10 minutes and preparation and drape plus angiography needling, the sevoflurane dose was reduced to 1 MAC. The ventilation was controlled to maintain the normocapnia status. The inspiration volume was 8 ml/kg with the respiration count, which maintained carbon dioxide between 30 and 35 mmHg. The measurements were done by a blind subject.
The heart rate, respiratory rate, non-invasive blood pressure, and oxygen saturation (pulse oximeter) were measured and recorded before and after anesthesia induction, before and after needling, and during measurements of pulmonary parameters and systemic blood pressure. All measurements were done by a single operator using the same device for each variable.
Having PCO2 as the most affected parameter by ventilation strategy, based on a pilot study of 10 patients, we assumed mean PCO2 of 34 and 39 during IPPV and spontaneous breathing, respectively. Thus, we determined that a total sample size of 60 would be sufficient to detect a 5 mmHg difference in PCO2 between the groups, estimating an SD of 5 mmHg, a power of 95%, and a significance level of 5%.
Data analysis was performed on the 60 patients’ data in two groups of 30 subjects by SPSS version 20.0 software. The used tests included the Chi-Square test, paired-sample t test, and independent-sample t test. A p value of less than 0.05 was considered statistically significant.
4. Results
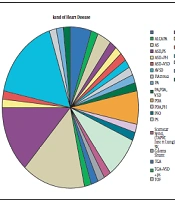
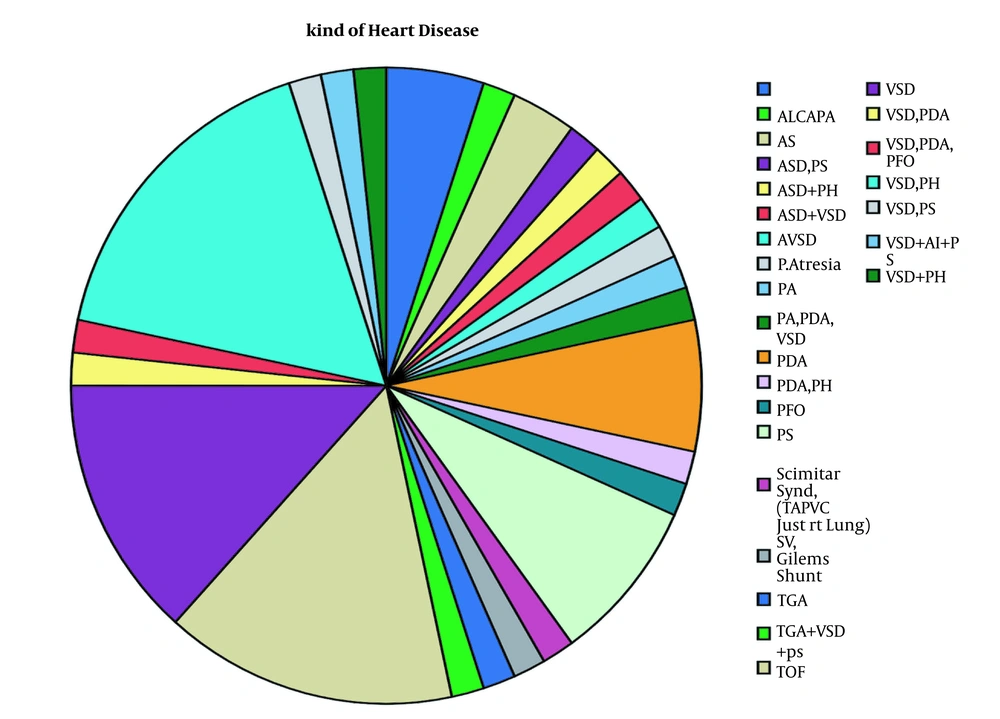
The mean age was 6.6 ± 3.3 months, ranging from 14 days to 12 months. Table 1 presents a summary of the baseline characteristics of the participants in the two groups, including gender, age, and past operation history. As displayed in Figure 2, the most common type of cardiac anomaly was a ventricular septal defect (VSD), along with pulmonary hypertension seen in 18.3%.
| Total (N = 60) | Intermittent Positive Pressure Ventilation (N = 30) | Spontaneous Ventilation (N = 30) | P-Value | |
|---|---|---|---|---|
| Gender | 0.301 | |||
| Male | 32 | 18 (60.0) | 14 (46.7) | |
| Female | 28 | 12 (40.0) | 16 (57.1) | |
| Age (mo) | 6.63 ± 3.40 | 6.21 ± 3.35 | 7.05 ± 3.46 | 0.348 |
| Past operation history | 59 | 2 (6.7) | 1 (3.3) | 0.574 |
aData are presented as mean ± SD or frequency (percentages).
Background diseases showed three cases with Down syndrome, one anemia case, and one patient with trans-esophageal fistula. Also, three cases had a positive surgical history. As shown in Table 2, there was no significant difference between the heart rates across the study. However, for systolic blood pressure, the measurement before anesthesia induction was significantly higher than that after anesthesia induction (P = 0.006). Also, the values during angiography were significantly lower than those in the initial stages (P = 0.021). However, after intubation and within recovery, there were no significant differences. For diastolic blood pressure, the values after induction were lower (P = 0.030) without significant changes in other stages. Besides, SPO2 was elevated after induction (P = 0.001) and was also higher after intubation (P < 0.001). Notably, it was not significantly different in other stages. On the other hand, the respiratory rate was significantly different across all stages, including after anesthesia (P = 0.002), after intubation (P < 0.001), during angiography (P = 0.017), and in recovery (P = 0.038). In all stages, the respiratory rate was reduced, except during recovery, which was elevated. In the recovery, there were five (8.3%) patients with nausea and vomiting, seven (12.5%) cases with pain, and eight (13.3%) patients with agitation.
| Mean ± SD | Minimum | Maximum | |
|---|---|---|---|
| Pre-anesthesia HR | 117.31 ± 15.07 | 78.00 | 155.00 |
| Pre-anesthesia systolic BP | 83.26 ± 12.56 | 60.00 | 110.00 |
| Pre-anesthesia diastolic BP | 51.07 ± 10.41 | 33.00 | 80.00 |
| Pre-anesthesia SPO2 percentile | 90.29 ± 9.80 | 55.00 | 100.00 |
| Pre-anesthesia RR | 28.15 ± 4.76 | 19.00 | 40.00 |
| Post-anesthesia HR | 116.34 ± 14.33 | 88.00 | 155.00 |
| Post-anesthesia diastolic BP | 49.37 ± 10.81 | 30.00 | 75.00 |
| Post-anesthesia SPO2 percentile | 91.91 ± 9.42 | 62.00 | 100.00 |
| Post-anesthesia RR | 26.09 ± 4.09 | 18.00 | 35.00 |
| After intubation HR | 118.14 ± 16.75 | 82.00 | 158.00 |
| After intubation systolic BP | 84.39 ± 13.21 | 55.00 | 115.00 |
| After intubation diastolic BP | 52.20 ± 10.81 | 30.00 | 77.00 |
| After intubation SPO2 percentile | 92.02 ± 9.53 | 55.00 | 100.00 |
| After intubation RR | 24.65 ± 4.77 | 15.00 | 38.00 |
| During angiography HR | 117.41 ± 16.23 | 85.00 | 170.00 |
| During angiography systolic BP | 80.90 ± 12.34 | 55.00 | 103.00 |
| During angiography diastolic BP | 50.71 ± 10.72 | 35.00 | 87.00 |
| During angiography SPO2 percentile | 89.53 ± 12.94 | 30.00 | 100.00 |
| During angiography RR | 28.76 ± 14.58 | 21.00 | 122.00 |
| During angiography PO2 | 80.37 ± 30.88 | 31.00 | 176.00 |
| During angiography PCO2 | 40.69 ± 6.67 | 25.00 | 66.00 |
| During angiography HCO3 | 20.80 ± 2.34 | 15.00 | 27.00 |
| Recovery HR | 118.12 ± 16.01 | 80.00 | 157.00 |
| Recovery systolic BP | 83.44 ± 12.35 | 58.00 | 110.00 |
| Recovery diastolic BP | 51.11 ± 10.20 | 33.00 | 72.00 |
| Recovery SPO2 | 90.82 ± 9.72 | 55.00 | 100.00 |
| Recovery RR | 29.20 ± 4.76 | 21.00 | 45.00 |
| Recovery time (min) | 40.00 ± 16.08 | 10.00 | 75.00 |
The differences between the two groups were assessed by the independent-sample t test. As shown in Table 3, SPO2 after anesthesia was higher in the IPPV group (P = 0.022), which was also higher after intubation (P = 0.019). During angiography, the PCO2 was lower in the IPPV group (P = 0.024). Besides, no statistically significant association was found between the two groups in nausea and vomiting, pain, and agitation (P > 0.05).
| Group | ||
|---|---|---|
| IPPV (Mean ± SD) | Spontaneous Ventilation (Mean ± SD) | |
| Pre-anesthesia HR | 118.07 ± 16.15 | 116.52 ± 14.10 |
| Pre-anesthesia systolic BP | 83.62 ± 12.41 | 82.90 ± 12.92 |
| Pre-anesthesia diastolic BP | 50.24 ± 10.11 | 51.90 ± 10.82 |
| Pre-anesthesia SPO2 percentile | 92.10 ± 6.59 | 88.36 ± 12.18 |
| Pre-anesthesia RR | 28.21 ± 4.52 | 28.09 ± 5.10 |
| Post-anesthesia HR | 117.87 ± 14.30 | 114.76 ± 14.44 |
| Post-anesthesia diastolic BP | 48.73 ± 11.79 | 50.03 ± 9.86 |
| Post-anesthesia SPO2 percentile | 94.73 ± 5.08 | 88.78 ± 11.96 |
| Post-anesthesia RR | 27.00 ± 4.00 | 25.09 ± 4.03 |
| After intubation HR | 120.73 ± 17.44 | 115.45 ± 15.86 |
| After intubation systolic BP | 86.00 ± 12.67 | 82.72 ± 13.77 |
| After intubation diastolic BP | 52.13 ± 10.49 | 52.28 ± 11.32 |
| After intubation SPO2 percentile | 94.93 ± 5.51 | 88.78 ± 11.87 |
| After intubation RR | 24.00 ± 4.89 | 25.36 ± 4.64 |
| During angiography HR | 118.83 ± 17.82 | 115.93 ± 14.57 |
| During angiography systolic BP | 81.23 ± 11.60 | 80.55 ± 13.26 |
| During angiography diastolic BP | 50.80 ± 11.25 | 50.62 ± 10.34 |
| During angiography SPO2 percentile | 91.10 ± 13.43 | 87.97 ± 12.46 |
| During angiography RR | 30.87 ± 20.11 | 26.55 ± 3.40 |
| During angiography PO2 | 81.63 ± 31.41 | 79.19 ± 30.91 |
| During angiography PCO2 | 38.61 ± 5.27 | 42.62 ± 7.32 |
| During angiography HCO3 | 20.90 ± 1.99 | 20.70 ± 2.66 |
| Recovery HR | 120.53 ± 17.63 | 115.44 ± 13.84 |
| Recovery systolic BP | 82.47 ± 12.97 | 84.52 ± 11.77 |
| Recovery diastolic BP | 49.60 ± 10.49 | 52.78 ± 9.78 |
| Recovery SPO2 | 93.07 ± 5.45 | 88.33 ± 12.58 |
| Recovery RR | 29.29 ± 5.03 | 29.09 ± 4.57 |
| Recovery time (min) | 39.50 ± 16.99 | 40.65 ± 15.17 |
5. Discussion
The maintenance of hemodynamic and respiratory physiological status during angiography leads to an increase in diagnostic accuracy. It is important in general anesthesia for children, which also increases the need for further assistance by cardiorespiratory support. At the same time, this can also affect accuracy.
The current research was a randomized controlled trial. The effects of IPPV versus spontaneous ventilation were determined on cardiorespiratory parameters in pediatric patients aged less than one year old undergoing angiography with general anesthesia.
According to the results, SPO2 and intubation plus PCO2 after anesthesia showed significant alterations among the study variables. Besides, SPO2 after anesthesia was significantly higher in the IPPV group than in the spontaneous ventilation group (P = 0.022). It was also higher after intubation in the IPPV group (P = 0.019). Further, PCO2 measurement was lower during angiography in patients under IPPV versus spontaneous ventilation (P = 0.024). Although some statistically significant associations were found between the two groups, the two methods of IPPV and spontaneous ventilation had similar effects. In other studies, Fujiwara et al. (17) and Fewell et al. (18) similarly reported no significant difference between the two methods.
The IPPV method would only lead to increased oxygen pressure and decreased carbon dioxide pressure in peripheral capillaries. Hence, IPPV may have some preferences over the spontaneous ventilation method, yet each of the methods may be used for pediatric cases under cardiac catheterization. Several other studies also demonstrated it in animal experimentations, such as Day et al. (19) and Pettifer et al. (20), who reported no difference in changes in hemodynamic and respiratory indices between the two IPPV and spontaneous ventilation methods in horses and parrots.
Overall, this study suggests that IPPV and spontaneous ventilation methods have the same effects on respiratory parameters, and each one may be used in children with different conditions under cardiovascular catheterization to enhance the accuracy with the reduction of alterations in cardiopulmonary parameters. Nevertheless, further studies with larger sample sizes and comparisons with other methods should be done to attain more definite comparable results.