1. Background
Nutrition is one of the most important aspects of care for premature infants (1). It is known that mothers’ inability to breastfeed their premature newborns leads to nutritional constraints for this high-risk group (2). On the other hand, in the gavage method, enteral feeding can meet the newborns’ nutritional needs. However, the immature gastrointestinal (GI) tract of these neonates can predispose them to some disorders, such as necrotizing enterocolitis (NEC), dysmotility, and feeding intolerance, concurrent with sepsis, hypoxic-ischemic encephalopathy (HIE), and other common neonatal problems (3).
NEC is a devastating complication of prematurity, associated with a high rate of mortality and morbidities, including short bowel syndrome, failure to thrive, cholestasis, and neurodevelopmental delay (4, 5). So far, many risk factors have been identified for NEC. Besides prematurity as the main risk factor, infection, inflammation, ischemia, feeding material, and hyperviscosity syndrome have been described as other contributing factors (6). In recent years, packed cell transfusion (PCT) in anemic preterm newborns has been also introduced as another risk factor for NEC at 48-72 hours post-transfusion (5).
The association between PCT and NEC, known as transfusion-associated NEC (TANEC), was first defined by McGrady et al. in 1987 among 33 preterm neonates, who were admitted to the neonatal intensive care unit (NICU) following an outbreak of NEC. Later, in 1998, Bednarek et al. evaluated the transfusion practices for preterm neonates in six NICUs and reported the lower incidence of NEC at centers with fewer transfusions, compared to those with a higher rate of transfusion (7). Since then, many researchers have published studies in this area, including a recent meta-analysis of seven non-randomized clinical trials (RCT), which showed that withholding nutrition in the peri-transfusion period reduced the rate of NEC. However, regarding the moderate quality of this study, the researchers suggested RCTs with adequate power for more accurate results (8).
A hypothetical mechanism has been suggested regarding the pathogenesis of TANEC, involving immune mechanisms responsible for the acute intestinal mucosal injury through passive transfusion of biological response mediators (e.g., donor antibodies) or activation of T-cell antigens in red blood cells (9). Other influential factors include the age of blood products, the severity of anemia in the neonate, and low levels of nitric oxides in stored red blood cells, resulting in the vasoconstriction of lower mesenteric vessels, reduction of blood flow, and mucosal injury (7). Evidence shows that oxygenation changes in the vascular bed of the mesenteric system cause TANEC during transfusion (5).
There are many studies with controversial results, evaluating the effect of withholding enteral feeding before, during, and after transfusion (10). However, until now, no specific protocol has been proposed for feeding preterm neonates during PCT. Regarding the high likelihood of need for PCT during hospitalization, access to a precise and standard guideline can help clinicians provide optimal care for these newborns and improve their outcomes. Although the majority of previous studies have focused on the correlation between PCT and NEC, little research has been conducted regarding the association between feeding intolerance (a preliminary marker of NEC) and PCT, especially in healthy premature neonates.
2. Objectives
With this background in mind, we hypothesized that feeding tolerance was related to PCT. Therefore, we assessed the association between PCT and feeding tolerance in healthy preterm newborns, admitted to the NICU of our hospital.
3. Methods
3.1. Study Design
This quasi-experimental interventional study was a randomized clinical trial among preterm infants, admitted to the NICU of Mofid Children's Hospital, Tehran, Iran, from April 2017 to May 2018. According to a study by Qi Lu et al. regarding the incidence of NEC (5), a total of 70 newborns were selected in this study (95% confidence level and 90% test power) and divided into two groups of 35 participants.
3.2. Study Sample
3.2.1. Inclusion Criteria
The inclusion criteria were as follows: 1) healthy premature infants with no abdominal distension, signs and symptoms of NEC, or sepsis; 2) birth weight < 1500 g; 3) gestational age < 32 weeks; 4) enteral feeding (by mouth or gavage); and 5) requiring PCT due to anemia. The neonate’s postnatal age was not a limitation in this study if enteral feeding was initiated.
3.2.2. Exclusion Criteria
With respect to the multifactorial nature of feeding intolerance and NEC in preterm infants, the exclusion criteria were as follows: 1) the presence of other factors causing feeding problems rather than PCT; 2) all sick newborns, including those with sepsis; 3) history of feeding intolerance or NEC; and 4) neonates without enteral feeding. Finally, only healthy preterm infants, who were hospitalized and required PCT for severe anemia, were enrolled in this study.
3.3. Randomization and Allocation
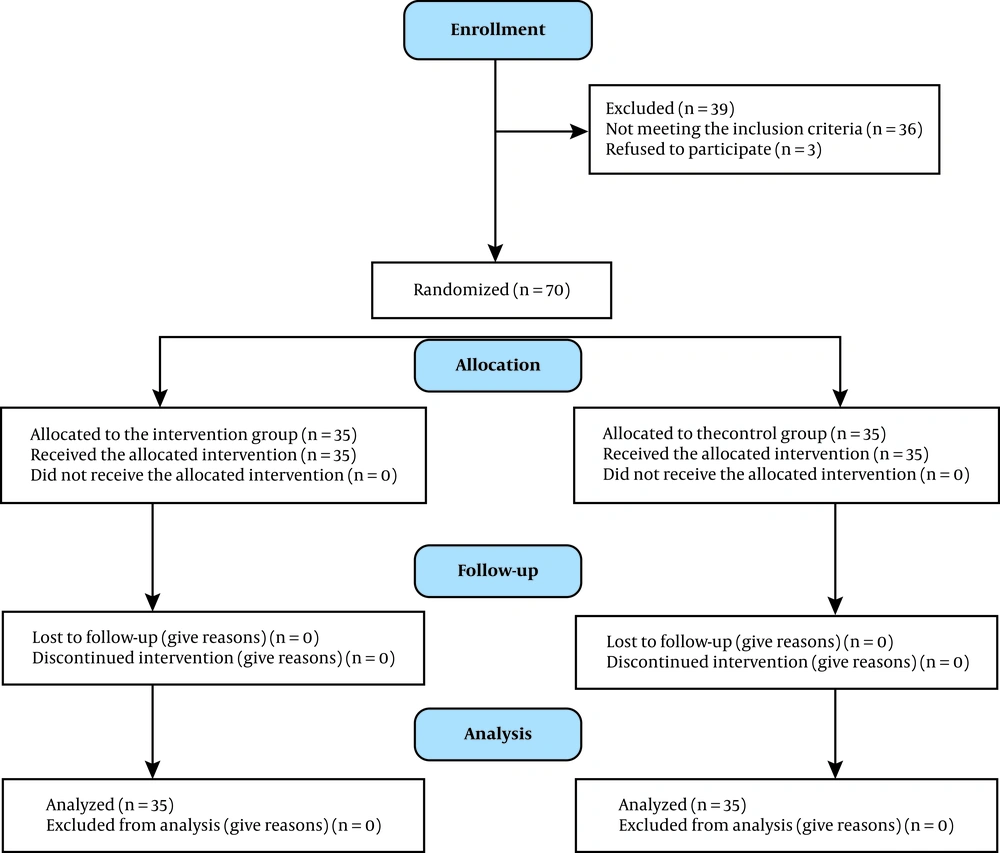
Block randomization was performed with blocks of the same size at the central randomization unit of the Neonatal Health Research Center. All newborns, who met the inclusion criteria, were assigned a number. Then, random numbers were presented to a speech therapy student, who randomly allocated the participants into the intervention and control groups. The eligible neonates were divided into two groups of 35 subjects each (35 subjects with code A and 35 subjects with code B). According to the neonatologist's order, feeding was withheld just during PCT for infants with code A, while liberal feeding was continued during PCT for infants with code B. Then we asked the aid researcher to measure the abdominal circumference 15 minutes before feeding time in two groups. In the next step the neonates' clinical nurse done the transfusion orders. The aid researcher measured the abdominal circumference 15 minutes after feeding time in two groups. If the abdominal circumference was more than 3-4 cm more than the last measure, the report of distention was recorded (11) (Figure 1).
3.4. Blinding
In this randomized, double-blind, controlled trial, the staff participants, care providers, assessors (except for one researcher running the intervention), and researchers were unaware of the participants’ random assignment to the groups during the study.
3.5. Interventions
PCT for the treatment of anemic patients was performed with respect to the patient’s general condition and cardiorespiratory status, based on the restrictive transfusion protocol. Since we only selected healthy preterm neonates without the need for oxygen supplement or mechanical ventilation, a hemoglobin level < 7 g/dL and a hematocrit level < 21% indicated PCT, based on the protocols. During the first 72 hours after transfusion, feeding tolerance was evaluated, based on the reports of the attending nurses and physical exams by the attending neonatologist or the NICU neonatal-perinatal fellow. The results of the exams were then compared between the two groups. As the standard protocols of feeding in preterm neonates admitted in our NICU, the patients participate in both groups of this research were feed preferential with breast milk with the volume of 10 - 20 cc/kg in the first 3 - 5 days of life, and with a daily increment of 20 cc/kg per day, gradually increase to a maximum volume of 150 - 180 cc/kg per day. Since we did not have access to the milk bank, for neonates without access to breast milk, we inevitably used preterm formula for feeding. When the feeding volume exceeded 50 cc/kg per day, we added a human milk fortifier to the breast milk for fortification. The protocol of feeding was the same in both intervention and control groups.
The initiation of feeding in Significant feeding intolerance, including gastric residual volume >30-50% of feeding volume, bilious vomiting, GI bleeding, abdominal distension (based on the abdominal circumference measurement before and after the feeding) and NEC, was compared between the two groups. If the abdominal circumference was more than 3 - 4 cm more than the last measure, the report of distention was recorded.
Despite the recommendations about the use of cytomegalovirus (CMV)-negative units and irradiated packed cells for very-low-birth-weight infants, due to the unavailability of these products, we conducted transfusion, regardless of the CMV status or unirradiated packed cells. The prescribed volume of packed cells for transfusion was 10 - 15 cc/kg, using a cross-matched product for the neonate, who was preferably not older than one week.
3.6. Outcomes
The number of transfusions was not a limitation in this study. However, patients with a history of multiple PCTs at different time points, with an interval of more than one week, were considered as new participants. Feeding intolerance was defined as a gastric residual volume > 30 - 50% of feeding volume, bilious vomiting, GI bleeding, and abdominal distension (detected by abdominal circumference measurement). Variables, including birth weight, gestational age, postnatal age, sex, hemoglobin and hematocrit levels before and after transfusion, and Rh blood group, were assessed in the intervention and control groups, and the correlations between these variables, feeding tolerance, and PCT were analyzed and compared.
3.7. Data Analysis
Data analysis was performed in SPSS version 20 (SPSS Inc., Chicago, IL, USA). Quantitative variables are expressed as mean and standard deviation (SD). Repeated measures ANOVA was used to evaluate differences between variables. P-value less than 0.05 was considered statistically significant.
3.8. Ethics Approval and Consent to Participate
The present study was extracted from a pediatrician’s thesis, submitted to Shahid Beheshti University of Medical Sciences, Tehran, Iran. All ethical considerations of the study were registered and approved by the institutional review board and the research ethics committee of Shahid Beheshti University of Medical Sciences (ethical code: IR.SBMU.RETECH.1395.1010; ClinicalTrials.gov Identifier: IRCT20200419047136N1). The newborns’ parents were informed of the study objectives and signed a written informed consent form. They were assured of the confidentiality of personal information and the voluntary nature of participation.
4. Results
A total of 70 preterm infants were included in this study. Thirty-five (50%) neonates were allocated to the intervention group and 35 (50%) neonates to the control group. Comparison of demographic data between the intervention and control groups is shown in Table 1. The mean gestational age, birth weight, and postnatal age were 30.13 weeks (SD = 2.1), 1245.71 g (SD = 256.736), and 17 days (SD = 23.42) in the intervention group and 29.97 weeks (SD = 2.32), 1169.43 g (SD = 201.508), and 15.46 days (SD = 15.84) in the control group, respectively; no significant difference was found between the two groups.
| Variables | Intervention Group, (NPO, N = 35) | Control Group, (PO, N = 35) | P-Value |
|---|---|---|---|
| Gestational age (w) | 30.11 ± 2.083 | 29.97 ± 2.256 | 0.784 |
| Postnatal age (days) | 17.06 ± 23.422 | 15.46 ± 15.844 | 0.739 |
| Birth weight (g) | 1245.71 ± 256.736 | 1169.43 ± 201.508 | 0.171 |
| Hemoglobin (pre-transfusion) | 8.274 ± 1.302 | 7.569 ± 1.1829 | 0.021 |
| Hematocrit (pre-transfusion) | 24.494 ± 3.6221 | 22.620 ± 3.6767 | 0.035 |
| Hemoglobin (post-transfusion) | 11.569 ± 1.8293 | 10.783 ± 1.4480 | 0.051 |
| Hematocrit (post-transfusion) | 33.811 ± 5.1211 | 31.954 ± 4.1299 | 0.100 |
| Sex | 0.595 | ||
| Male | 20 (57.1) | 20 (57.1) | |
| Female | 15 (42.9) | 15 (42.9) | |
| Delivery mode | 0.239 | ||
| C/S | 32 (91.4) | 29 (82.9) | |
| NVD | 3 (8.6) | 6 (17.1) | |
| Rh blood group | 0.368 | ||
| A | 8 (22.9) | 6 (17.1) | |
| B | 18 (51.4) | 13 (37.1) | |
| AB | 5 (14.3) | 10 (28.6) | |
| O | 4 (11.4) | 6 (17.1) | |
| History of transfusion | 0.270 | ||
| Yes | 5 (14.3) | 8 (22.9) | |
| No | 30 (85.7) | 27 (77.1) | |
| Feeding material | 0.037 | ||
| Breast milk | 11 (31.4) | 8 (22.9) | |
| Breast milk fortifier | 3 (8.6) | 7 (20) | |
| Breast milk + formula | 11 (31.4) | 3 (8.6) | |
| Formula | 10 (28.6) | 17 (48.6) | |
| Feeding volume | 0.197 | ||
| Complete feeding | 6 (17.1) | 10 (28.6) | |
| Incomplete feeding | 29 (82.9) | 25 (71.4) | |
| History of mechanical ventilation | 0.401 | ||
| Yes | 13 (37.1) | 11 (31.4) | |
| No | 22 (62.9) | 24 (68.6) |
Comparison of the Demographic Characteristics of the Newborns in the Intervention and Control Groupsa
The mode of delivery was caesarian section (CS) in 91.4% (n = 32) and 82.9% (n = 29) of the subjects in the intervention and control groups, respectively, with no significant difference (P = 0.239). The mean levels of hemoglobin and hematocrit before transfusion were 8.25 mg/dL and 24.4 mg/dL in the intervention group and 7.5 mg/dL and 22.6 mg/dL in the control group, respectively. A significant difference was observed between the two groups regarding hemoglobin and hematocrit levels (P = 0.021 and P = 0.035, respectively); based on the results, the levels of hemoglobin and hematocrit were lower in the control group. Also, the mean post-transfusion levels of hemoglobin and hematocrit were 11.5 mg/dL and 33.8 mg/dL in the intervention group and 10.78 mg/dL and 31.95 mg/dL in the control group, respectively, without any significant difference between the groups (P = 0.05 and P = 0.1, respectively).
As shown in the table, there was no significant difference between the two groups in terms of sex (P = 0.595), gestational age (P = 0.784), birth weight (P = 0.171), postnatal age at the time of transfusion (P = 0.739), history of transfusion (P = 0.270), mechanical ventilation (P = 0.401), neonate’s Rh blood group (P = 0.368), and feeding material (i.e., breast milk alone, formula, breast milk, and fortifier) (P = 0.197). The most common Rh blood group was B+ in both groups (51.4% and 37.1% in the intervention and control groups, respectively). Moreover, the results showed that 60% and 57.2% of preterm neonates in the intervention and control groups were formula-fed, respectively. Overall, the high percentage of neonates, deprived of their mother’s breast milk, is an alarming sign for healthcare providers to increase their efforts for enhancing the rate of breastfeeding.
Regarding feeding tolerance, we monitored the patients’ feeding tolerance at 24 - 72 hours post-transfusion. As shown in Table 2, feeding tolerance was compared between the intervention and control groups at 1-3 days after transfusion. The number of patients with feeding intolerance was limited in this study, and there was no feeding intolerance in 32 (91.2%), 33 (94.73%), and 34 (97.1%) neonates of both groups at 24, 48, and 72 hours post-transfusion, respectively. Also, there was no significant difference between the two groups in terms of feeding tolerance at 24, 48, and 72 hours post-transfusion (P = 0.663, P = 0.693, and P = 0.754, respectively).
Despite the higher number of exclusively formula-fed neonates in the control group versus the intervention group (48.6% vs. 28.6%; P = 0.037), feeding intolerance was not significantly different. Moreover, in this study, we compared the feeding tolerance of newborns in each group at 1-3 days post-transfusion. Feeding tolerance had no significant correlation with variables, including postnatal age, hemoglobin level, and hematocrit level pre- and post-transfusion (P > 0.05).
| Feeding Tolerance | Intervention Group (N = 35) | Control Group (N = 35) | P-Value |
|---|---|---|---|
| First 24 hours | 0.663 | ||
| Yes | 32 (91.4) | 32 (91.4) | |
| No | 3 (8.6) | 3 (8.6) | |
| 24 - 48 hours post-transfusion | 0.693 | ||
| Yes | 33 (94.3) | 33 (94.3) | |
| No | 2 (5.7) | 2 (5.7) | |
| 48-72 hours post-transfusion | 0.754 | ||
| Yes | 34 (97.1) | 34 (97.1) | |
| No | 1 (2.9) | 1 (2.9) |
. Comparison of Feeding Tolerance at 1-3 Days Post-transfusion Between the Intervention and Control Groupsa
5. Discussion
The reduced iron storage of preterm neonates at the time of birth, inability to breastfeed the infant within the first days or weeks of life, frequent blood sampling, and decreased level of erythropoietin (leading to anemia) are associated with frequent PCT during the hospitalization of premature infants (12). Although PCT is a lifesaving treatment, it can cause some complications for the newborn. A recently known complication of PCT is NEC and the associated feeding problems. To reduce these complications, withholding enteral nutrition during transfusion has been suggested for preterm newborns (8).
In this clinical trial, we aimed to evaluate the correlation between PCT and feeding tolerance in healthy preterm infants. Our results showed that discontinuing feeding had no effects on the feeding tolerance of newborns. It seems that the liberal feeding protocol, regardless of the time of transfusion, is safe for these healthy premature newborns. To eliminate the effects of other risk factors for feeding intolerance, such as sepsis, NEC, and HIE, we only included healthy premature infants, without any history of the mentioned problems. In this regard, some recent studies have shown that 25 - 35% of NEC cases are related to PCT (13). Although substantial research has been conducted in this area, the results are inconclusive so far, and there is no standard protocol for feeding preterm infants during transfusion (14).
In line with the present research, a case-crossover study by Le VT et al. on NEC, milk fortification, and milk volume increase showed that milk fortification and volume had no associations with GI problems in 63 preterm infants < 32 weeks of gestation (15). Conversely, some other studies, including the one conducted by Alfaleh et al., showed that PCT was associated with a lower rate of NEC in preterm infants (16). Also, in the present study, the mean age of the newborns in the intervention and control groups was 17 and 15 days, respectively. It should be noted that we only included healthy preterm infants, as systemic problems, such as respiratory distress syndrome (RDS), sepsis, and need for mechanical ventilation or surfactant therapy, which can cause feeding intolerance and confound the results, are more probable during the first days of life in premature patients. Overall, most of our patients were older than two weeks and had a better general condition for being included in the study.
Additionally, many studies have indicated the impact of delivery mode on the outcomes of preterm newborns. Intraventricular hemorrhage, HIE, and RDS are neonatal conditions, affected by the mode of delivery. However, our findings did not show any significant difference in the rate of NEC or feeding intolerance after transfusion in preterm newborns, delivered by CS or natural vaginal delivery (NVD) (P = 0.239); this result is consistent with a study by Monika Bajaj (P = 0.37) (17). Despite the clinicians’ emphasis on the importance of feeding preterm infants the expressed breast milk of their own mothers, the overall rate of breastfeeding in our study population was 42%. Generally, our hospital is a referral center without nursery, and most of the patients are referred from remote areas. Therefore, we did not have access to breast milk at all times, and due to the lack of a milk bank in our hospital, the use of formula as an alternative was justified. Regardless of the more frequent use of formula in the control group, the rate of feeding intolerance was similar between the two groups.
There are inconclusive recommendations about the duration of withholding nutrition and PCT. Some studies have suggested discontinuing enteral feeding in patients one to four hours before transfusion, while some studies have not recommended feeding during or after transfusion, even after 24 hours (7). We withheld feeding of neonates in the intervention group only during transfusion and monitored all cases in the intervention and control groups at 24 - 72 hours post-transfusion, based on the definition of TANEC, that is, development of NEC within 48-72 hours after transfusion. As mentioned earlier, we did not find any significant differences in terms of feeding intolerance between the two groups.
Anemia, as an underlying risk factor for TANEC associated with subclinical intestinal mucosal injury, precedes the overt clinical signs and symptoms of enterocolitis. The possible mechanisms include immature re-oxygenation injury in the anemic gut and the splanchnic vascular bed and immune mechanisms similar to those seen in transfusion-related acute lung injury (TRALI) (18). Therefore, PCT may not be a primary cause of intestinal injury, but can be another risk factor for an underlying mucosal injury due to severe anemia. According to this hypothesis, the level of hemoglobin and severity of anemia may influence the incidence of feeding intolerance.
Some studies examined the effect of hemoglobin level on the occurrence of TANEC and reported its significant effect on the incidence and severity of NEC. These studies concluded that the lower level of hemoglobin is associated with an increased risk of NEC (19). In our study, the level of hemoglobin in the control group was significantly lower than that of the intervention group (7.56 mg/dL vs. 8.27 mg/dL; P = 0.021); however, there was no significant difference in feeding intolerance between the groups. This finding may be attributed to the insignificant difference in the level of hemoglobin between the two groups.
Moreover, a retrospective cohort study by Cris Derienzo et al. on 148 very-low-birth-weight infants evaluated the association of TANEC with this condition, regardless of blood transfusion. They found that TANEC was more common in smaller premature newborns with lower levels of hematocrit before transfusion; in other words, more severe anemia in premature infants was associated with a higher incidence of TANEC. They recommended maintaining higher levels of hemoglobin and hematocrit as protective factors in infants at a higher risk of NEC (20). Also, in a study by Ravi M. Patel et al. on very-low-birth-weight infants, aged 0-5 days, severe anemia (not PCT) was associated with NEC (21). However, the mean age of the neonates in their study was lower than ours, and the severity of anemia did not have any significant effects on the feeding intolerance.
Overall, the prolonged hospitalization of preterm infants is associated with the repeated need for PCT. In our study, 5 (14.3%) neonates in the intervention group and 8 (22.9%) cases in the control group had a history of PCT, which had no significant effects on the rate of feeding intolerance. It is unclear whether repeated transfusion has an additive effect on the feeding intolerance or NEC; however, the interval between repeated transfusions is important. In this regard, a study by Yu-Cheng Wang showed that the number of RBC transfusions was higher in patients with NEC (22).
The main limitation of the present study was the low sample size. Nevertheless, the methodology of this study was suitable, considering the limited sample size.
5.1. Conclusions
Although there are major concerns regarding the association of NEC with severe anemia and PCT in preterm infants admitted to NICUs, precise timing of nutrition discontinuation for neonates requiring PCT has not been determined yet. While almost all previous studies on the association between PCT and NEC have evaluated sick preterm infants, there are few studies assessing feeding intolerance (not NEC) following PCT in healthy premature neonates with a stable condition. The results of our RCT showed that continuing nutrition during PCT for healthy preterm neonates with a good general condition did not cause feeding intolerance, and withholding feeding was not necessary; therefore, continued breastfeeding seems to be a safe option. However, this result cannot be generalized to sick preterm infants with other underlying risk factors for NEC, sepsis, or GI problems.