1. Introduction
Granulomatosis with polyangiitis (GPA), formerly known as Wegener's disease, is a kind of anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV), which involves small-to-medium-sized vessels (1). Besides, GPA is a necrotizing granulomatous inflammation, usually involving the upper and lower respiratory tract, and also causing necrotizing glomerulonephritis (2). The involvement of the heart and Gastrointestinal Tract (GI) tract in GPA is unusual, and these are atypical places of this type of vasculitis (3, 4). The cardiac manifestations are not often clinically obvious, but they can increase the mortality rate (5). Although the involvement of GI is rare, perforation is one of the frequently described pathologies in GPA patients (4). We herein report a 14-year-old girl with a newly diagnosed GPA after COVID-19 infection who presented with ST-elevation MI, GI perforation, and intracranial hemorrhage.
2. Case Presentation
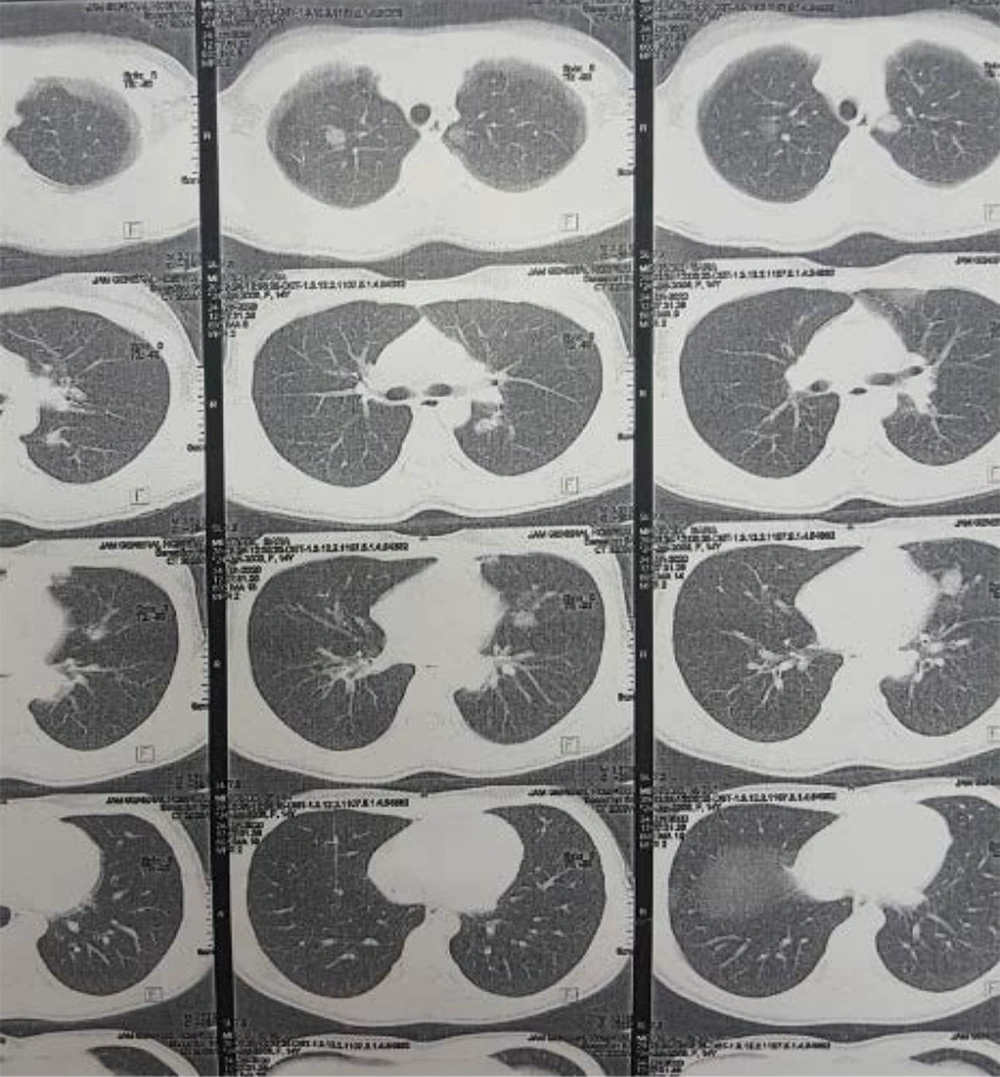
The patient was a 14-year-old girl who presented with symptoms of respiratory tract infection in June 2020 and was treated with the diagnosis of acute. Three weeks later, she developed the symptoms of pain and swelling in different joints, with migratory patterns and occasional fever. Because of these complaints, the patient was admitted to a hospital for diagnostic evaluations. According to her recent respiratory symptoms and COVID-19 outbreak, she was evaluated for COVID-19. The RT-PCR and Ig M were negative, but the IgG level was positive (IgG = 4.2). A chest CT scan (Figure 1) was performed, which showed bilateral multifocal nodular infiltrates with halo sign view, which was suggestive of vasculitis in particular GPA. Also, there were subpleural consolidation foci and centrilobular nodules, which could be in the context of bronchopneumonia in Coronavirus infection background. According to sinusitis history, chest CT scan evidence, and arthritis, the patient was further evaluated for GPA. Paranasal sinuses CT scan was performed. The findings suggested acute sinusitis. The patient underwent a sinus biopsy, and the report showed necrotizing sinusitis and vague granulomatosis reaction compatible with GPA. The results of BAL and fungal cultures were negative, but diffuse nodularity was seen in bronchoscopy. A biopsy was done with the results of inflammation and necrosis, according to GPA. She had leukocytosis with a shift to the left, anemia, thrombocytopenia, elevated ESR and CRP, positive PR3-ANCA, and RF (Table 1). Abdominopelvic ultrasound was normal. Upper GI endoscopy only showed antral gastritis. Echocardiography was reported as mild TR with 60 - 65% EF.
| Lab. data | Value |
|---|---|
| WBC (× 1000/mm3) | 12.7 |
| Neut (%) | 72 |
| Lymph (%) | 16 |
| RBC (Mill/mm3) | 4.2 |
| Hb (g/dL) | 11.4 |
| MCV (fL) | 77 |
| Plt (× 1000/mm3) | 487 |
| ESR (mm/h) | 108 |
| CRP (mg/L) | 150 |
| BUN (mg/dL) | 36 |
| Cr (mg/dL) | 0.6 |
| Na (mmol/L) | 135 |
| K (mmol/L) | 4.2 |
| Ca (mg/dL) | 8.4 |
| AST (U/I) | 20 |
| ALT (U/I) | 20 |
| LDH (U/L) | 909 |
| U/A | Nl |
| C ANCA | Positive |
| P ANCA | Negative |
| ANA | Negative |
| RF | 3+ |
| Anti CCP | Negative |
| ACE (U/L) | 25 |
| CPK (U/L) | 40 |
| CKMB (µg/L) | 10 |
| Procalcitonin (ng/mL) | 0.27 |
Abbreviations: WBC, white blood cell; Neut, neutrophil; Lymph, lymphocyte; RBC, red blood cell; Hb, hemoglobulin; MCV, mean corpuscular volume; Plt, platelet; ESR, erythrocyte sedimentation rate; CRP, -reactive protein; BUN, blood urea nitrogen; Cr, creatinine; Na, sodium; K, potassium; Ca, calcium; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDH, lactate dehydrogenase; U/A, urine analysis; ANCA, antineutrophil cytoplasmic antibody; ANA, antinuclear antibody; RF, rheumatic factor; Anti CCP, anticyclic citrullinated peptide antibody; ACE, angiotensin-converting enzyme; CPK, creatine phosphokinase.
The patient was treated with methylprednisolone pulse 500 mg daily for three days, IVIG 20 g daily for five days, and finally, cyclophosphamide pulse. She was discharged with prednisolone tablets 60 mg daily and recommended to refer one month later to receive the second pulse of cyclophosphamide.
After discharge from the hospital, her drugs were changed to prednisolone tablets 20 mg daily, azathioprine 50 mg daily, and MMF daily. Within two weeks, excessive fatigue and weakness occurred. The patient's abdominal pain intensified, and she developed skin lesions in the extremities. Her new laboratory data showed leukocytosis with a shift to the left, anemia, thrombocytosis, and elevated acute phase reactants. Thus, she was hospitalized again with a disease flare-up diagnosis and treated with methylprednisolone pulse 1 g. She developed chest pain during hospitalization. Then, ECG showed anterior ST-elevation MI. Echocardiography was reported as 40% EF, apical segment hypokinesia, and mild MR. Cardiac enzymes elevated (troponin T = 504 and troponin I = 11.36). Thus, she was emergently referred to our hospital for therapeutic procedures.
In the catheterization ward, percutaneous coronary intervention (PCI) on LAD (left anterior coronary artery) and glycoprotein IIb/IIIa injection were done. In addition to the LAD involvement (cut from mid part), the left circumflex artery (LCX) was ectatic in the distal part, and the diagonal artery had significant stenosis. Then, she was transmitted to the cardiac care unit (CCU) and treated with heparin, aspirin, Plavix, carvedilol, rosuvastatin, and losartan. She also received a corticosteroid stress dose. After performing necessary cardiac treatments and considerations for the patient, she was transferred to the pediatric ward for underlying disease control by a pediatric rheumatologist. During admission, she experienced severe postprandial abdominal pain. Abdominopelvic CT angiography with IV contrast showed enhancement areas adjacent to the portal vein, linear hypodense areas in both kidneys, a hypodense area in the liver, some lymph nodes, and mild pelvic fluid.
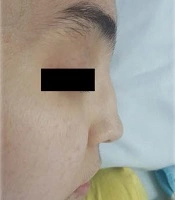
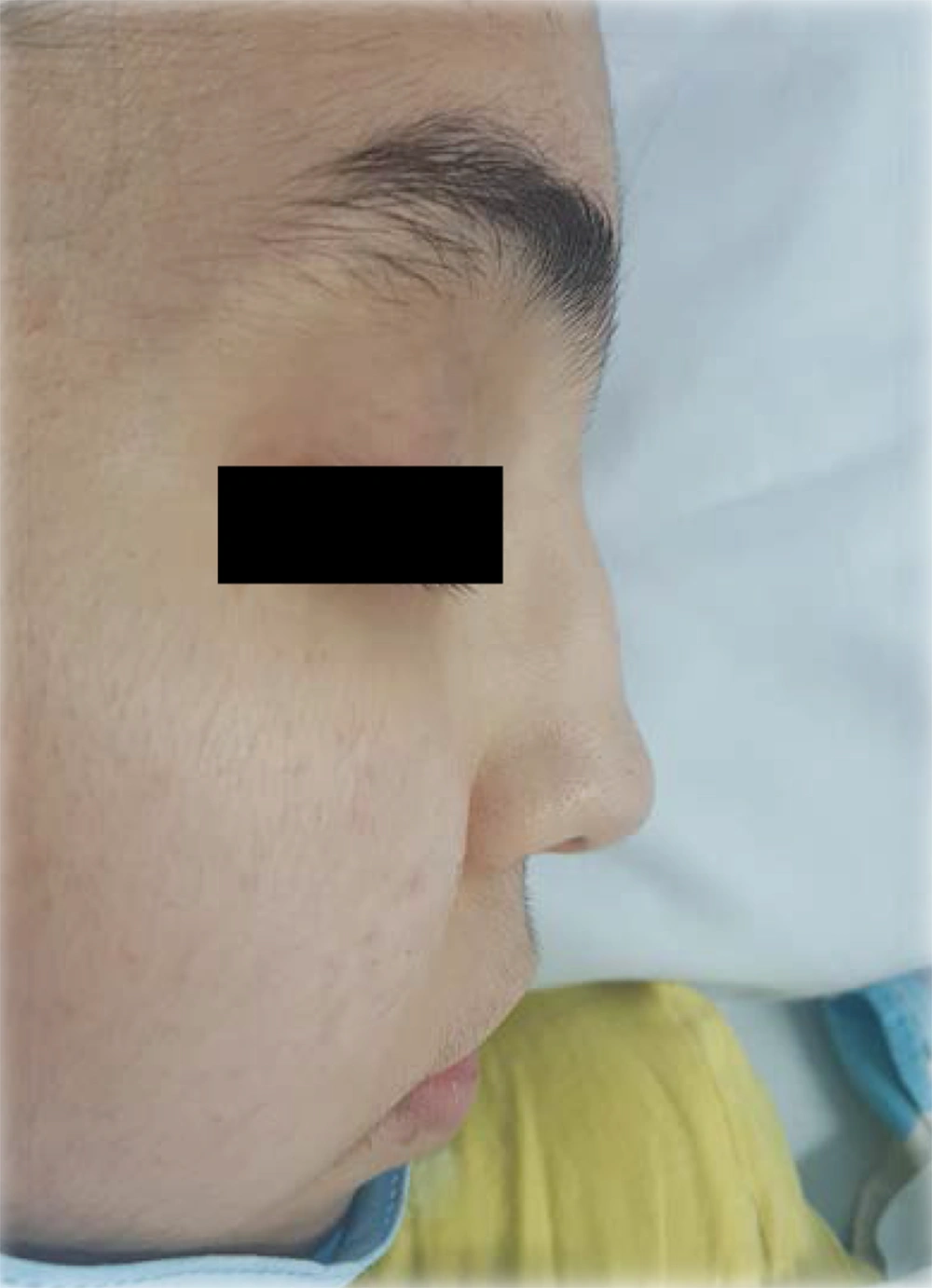
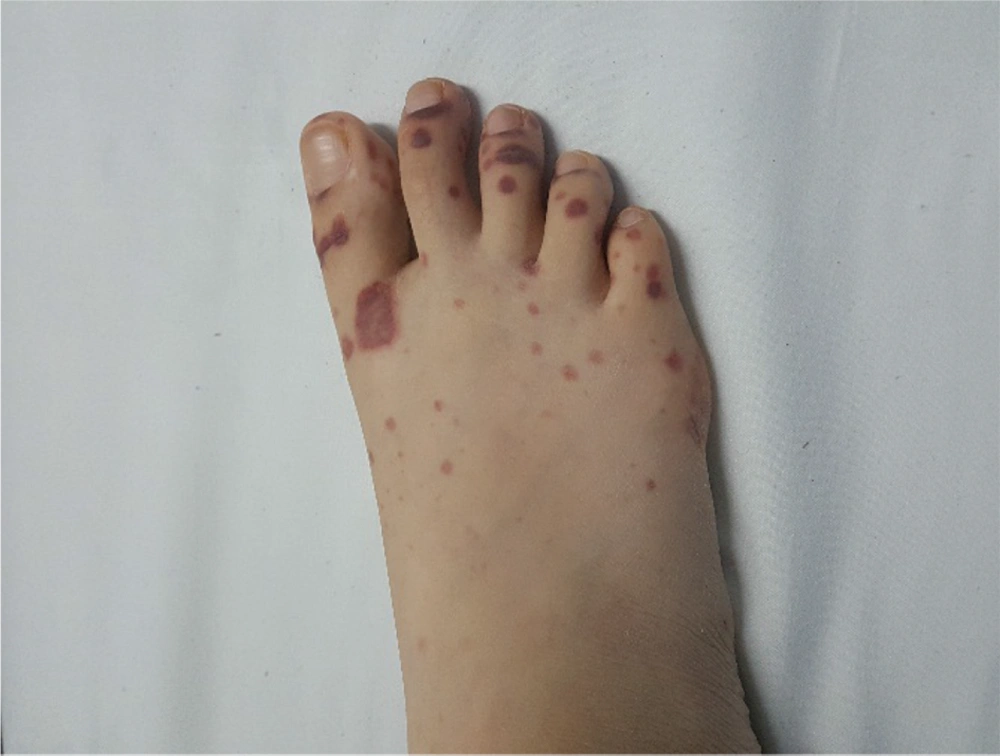
The patient was reevaluated again. In her history, she had complained of recurrent frontal headaches in the last two years. She recently had one episode of epistaxis. The parents were not consanguineous. The patient had a healthy older sister. The patient weight was 40 kg, and her height was 167 cm. On physical examination, saddle nose deformity was diagnosed in her (Figure 2). Furthermore, she had palpable petechia and purpuric rash on her lower extremities (Figure 3). Based on the patient's laboratory data, antiphospholipid antibodies, coagulation study, and hepatitis B tests were normal. She received a packed cell because of anemia. We decided to treat her with rituximab 375 mg/m2 weekly for four doses, prednisolone tablets 1 mg/kg per day, and MMF for remission induction. We started nifedipine 10 mg daily to relieve the abdominal pain. She also continued her cardiac treatment under the observation of our cardiologist.
During admission to receive the second dose of rituximab, her abdominal pain exacerbated. On physical examination, the abdomen was soft, but there was generalized abdominal tenderness. According to a surgery consultation and sub-diaphragmatic air on chest X-ray, she was transferred to the operation room. The post-operation diagnosis was generalized peritonitis due to small bowel perforation in the jejunum (1 cm) and abscess formation. So, small bowel resection and anastomosis, and abscess drainage were done. Medical therapy continued. On the 10th day after surgery, ultrasound showed a collection (56 × 19 mm) near to splenic curvature. Concerning the continuation of abdominal pain along with this evidence, the collection was discharged on the guide of sonography. But, her condition deteriorated. She was afebrile but had tachycardia (HR = 130/min). On abdominal examination, her abdomen became tense. Therefore, explorative laparotomy was performed again. The post-operation diagnosis was peritonitis due to anastomosis leakage, and after being evacuated of her abdomen from bile leakage, jejunostomy placement was done. The patient's condition became better after the surgery, and treatment with steroids, antibiotics, and cardiac drugs continued.
Six days after the second surgery, the patient had a tonic-clonic seizure that lasted for five minutes and was stopped by the administration of IV diazepam. There was no focal sign on the examination. Although the patient was receiving TPN, she was suffering from electrolyte disturbances. Hypocalcemia and hypomagnesemia were detected in laboratory data after the seizure. Nevertheless, brain MRI and MRA were performed and revealed signal increases in cortical and subcortical areas of the posterior brain on T2 FLAIR sequences. These findings might be due to posterior reversible encephalopathy syndrome (PRES) as a result of hypertension or because of vasculitis in the background of her underlying disease. Three weeks after her seizure, despite the acceptable condition, she suddenly developed hemiparesis. Her brain MRI showed an intracranial hemorrhage. She underwent brain surgery immediately, but unfortunately, she died.
3. Discussion
According to acute sinusitis, the evidence of chest CT scans, the results of urine Pr/Cr ratio, positive PR3-ANCA, and sinus biopsy results, the patient fulfilled the EULAR/PRINTO/PRES criteria (6). The exact etiology of GPA is unknown. However, it is presumed in genetically predisposed patients. Some different triggers, such as infections can lead to the production of ANCA, which causes tissue inflammation and vascular injury (7). Lung involvement occurs in most GPA patients, and respiratory pathogens are largely implicated in the pathogenesis of this disease. One proposed mechanism for the development of ANCA suggests the initiation of autoimmune response done by peptides that are complementary to peptides in the target antigens, such as PR3 (8). Particularly, Staphylococcus aureus has peptides that mimic cPR3, that can initiate or exacerbate GPA. Entamoeba histolytica and Ross River virus are other pathogens with peptides that can mimic PR3 (9). Similarly, activated autoantibodies and T-cells in coronavirus may attack the host cell antigens, which mimic the viral antigen (10). The low titer of virus antigen is detected in the cytokine storm phase in favor of this mechanism. Similarly, ANCA may be synthetized in susceptible patients triggered by a coronavirus. The cytokines from activated T-cells (INFy, TNFα) trigger the other inflammatory cells such as macrophages, neutrophils, and endothelial cells with cytokines secretion. Consequently, this cycle repeats again and again so that it leads to a cytokine storm. On the one hand, activated endothelial cells with cytokines, combined with direct inflammation of endothelial cells by ACE2 receptor of coronavirus, may cause vasculitis- like, or these may be true vasculitis in addition to the dysfunction of endothelial cells in a cytokine storm situation (11). On the other hand, in both PGA and cytokine storm situations in coronavirus disease, neutrophil extracellular traps (NETs) may have a significant role. The NET overexpressed MPO and PR3 antigens lead to the production of ANCAs in PGA. Thus, NETs have a significant defensive role against viral infections. However, NET could trigger the inflammatory and immune response in virus infection with the overexpression of inflammation (12, 13). Therefore, the generation of NET by stimulated neutrophils with coronavirus in cytokine storm situation may trigger the production of ANCA besides the endothelial vascular inflammation.
In our patient, the onset of the disease was after recent infection with COVID-19. Whether coronavirus could be a triggering environmental factor for the presentation of ANCA-positive vasculitis or whether it was just a coincidence is a big question. Recent papers have discussed the role of the coronavirus in vasculitis (14-16).
Cardiac involvement of GPA is rare, and based on these issues, it was first described by Wegener in 1936 (17). This complication can occur with different presentations such as myocarditis, pericarditis, endocarditis, coronary involvement, valvular abnormalities, conduction disorders, heart failure, cardiomyopathy, and myocardial infarction (4, 18-20). The exact rate of cardiac involvement, especially in pediatric GPA, is not clear, and this rate differs from one study to another, but it is clinically rare (21, 22). Although GPA can cause heart abnormality, in many cases, cardiac involvement has no signs (23). Several case reports have shown that myocardial infarction can occur in GPA, and it is silent in many cases. Myocardial infarction sometimes, occurs when other manifestations of GPA are under control with immunosuppressive therapy (18, 24-26). In a cohort study on 504 adult GPA patients, Avina-Zubieta et al. concluded that these patients have a significantly increased risk of myocardial infarction. Thus, it is important to monitor this complication and modify risk factors, especially at the beginning of diagnosis (27).
For the management of myocardial infarction in GPA, in addition to the use of cardiac drugs and percutaneous coronary intervention (PCI), if needed, systemic corticosteroids and immunosuppressive therapy are essential. Recently, biological drugs, especially rituximab, seem to have a good effect (19, 28). As a result of these, we decided to treat our patient with rituximab plus high-dose oral prednisolone.
Gastrointestinal involvement in GPA is a rare complication, and ranges from 0 - 26% in adult patients (29-31). In a cohort study done on 28 pediatric patients with GPA, one-third of them had this involvement (32). Although the GI system involvement can be the first symptom of GPA, it usually happens years after the onset of the disease and the beginning of treatment (33, 34). In the setting of GPA, every part of the GI tract can be affected (30). In a literature review of 50 cases with GI involvement of GPA from 1982 to June 2016, it was concluded that every part of the GI tract could be affected by GPA. Nevertheless, most of the reported cases had multiple locations’ involvement, and the small intestine was the most common site (35). Different GI symptoms might occur in GPA; for example, abdominal pain, hemorrhage, peptic ulcers, pancreatitis, and perforations. But, intestinal perforations are uncommon in the course of this disease (30).
In GPA, the underlying vasculitis itself could lead to GI perforation. Treating with immunosuppressive drugs, as known, is the cornerstone of GPA treatment. On the other hand, these kinds of drugs, especially glucocorticoids, could be one of the causes of GI perforations. It is very difficult to differentiate between these two causes (36). However, in a study done by Skaife et al., intestinal perforations occurred in a patient with GPA as the presenting sign of disease without receiving any immunosuppressive treatment (37).
During a literature review done by Akbulut, vasculitis was reported in 61.5% of the perforated areas in histopathology. Thus, he suggested that vasculitis plays a more important role than glucocorticoids in the development of intestinal perforation in GPA patients (30), although this issue is very complex, and our information is incomplete. However, it is clear that we need to continue immunosuppressive drugs to control the disease and prevent its relapses.
Neurologic involvement is reported approximately in 20 - 50% of patients with GPA, but central nervous system manifestations are not very common in these patients (38). There are different pathogenic mechanisms for CNS involvement in GPA: (1) adjacent invasion of granuloma from extracranial sites; (2) remote intracranial granuloma; and (3) CNS vasculitis (39). Whether our patient's seizure was due to electrolyte disturbance or brain involvement in her disease process is another question. Her death due to intracranial hemorrhage made the brain involvement play a further important role.