1. Background
Tooth decay is an infectious and multifactorial oral disease caused by the metabolic interaction of caries-causing oral flora with dietary fermentable carbohydrates on the tooth surface in the diet over time. This interaction can cause acid production, demineralization, and caries cavities at the tooth surface. Dental caries are the result of the dynamic process of demineralization and remineralization of dental materials. This process occurs several times a day and is modified by several factors, including oral hygiene, diet, genetics, dental anatomy, intrinsic resistance of tooth structure, saliva flow, buffering capacity, number and type of microbial flora, and fluoride use (1, 2)). Streptococcus mutans (S.M.) and Lactobacillus acidophilus (L.A.) are the main bacterial strains involved in the decay process as causative and progressive factors, respectively (3, 4). There are different mechanisms of dental caries prevention that are based on limiting demineralization caused by caries-causing bacteria. These methods include metabolism alteration and growth inhibition of pathogens, increasing tooth surface resistance to demineralization, and increasing pH in dental plaque (5). It is well established that there is an inverse relationship between enamel fluoride content and tooth decay prevalence (6). Fluoride ions increase the compressive strength of enamel crystals, so acid resistance of enamel is increased. Primary carious lesions are remineralized with a similar mechanism. Also, fluoride has antimicrobial activity (7).
The use of low-fluoride products such as toothpaste is recommended for everyone. High-concentration, topical fluoride products such as varnish and gel are used periodically for children and adults with a high risk of caries (8, 9). The varnish adheres to the tooth enamel and gradually releases the fluoride. So, the tooth is exposed to fluoride for a long time period (10). Safety, efficacy, and no need for long-term treatment are the prominent features of varnishes (10). Easy use of fluoride varnish makes it suitable for the treatment of very young and non-cooperative patients (11). In a systematic review of relevant clinical trials, it has been recommended to apply sodium fluoride varnish twice a year to control caries (12).
High levels of fluoride ions in saliva (≥ 12,000 ppm) are toxic for some bacteria such as S.M. It has been reported that growth inhibition of S.M. has continued for several weeks after one time using topical fluoride (13). So far, several previous studies have been conducted on the antimicrobial properties of varnishes. For example, in vitro study of the antibacterial effects of xylitol-containing varnishes against S.M. or clinical study about different varnish products on salivary S.M. count (14-16). In these studies, less attention has been paid to the antimicrobial properties of varnishes against L.A. Because the structural and metabolic characteristics of each microorganism are different from those of other microorganisms, the results of studies that are related to S.M. cannot be generalized to L.A. (4). Another issue is that the formulation properties, concentration and release behavior of fluoride vary in different varnishes and therefore differentiate the antibacterial effects of these products (17).
2. Objectives
The present in vitro study was designed to evaluate the antibacterial activity of three common sodium fluoride varnishes with different compounds on both S.M. and L.A.
3. Methods
The present research is an experimental, in vitro study in which the antimicrobial effects of three fluoride varnishes Polimo, FluoroDose, and MI varnish, on S.M. and L.A. were investigated in the Microbiology Laboratory of Mazandaran University of Medical Sciences. The specifications of these varnishes are given in Table 1. To perform the experiment, the lyophilized form of two standard strains of S.M. (No. 1683PTCC) and L.A. (No. 1643PTCC) were provided from Iran Scientific and Industrial Research Organization. Using the basic products of QUELAB laboratories INC, Canada, we prepared bacteriological culture media, including MSA (Mitis Salvarius agar) containing sucrose and bacitracin to remove contaminants, MRS (Man, Rogosa, Sharpe) agar and broth with 0.05% W/V solution of cysteine chloride, Müller blood agar (Müller-Hinton agar supplemented with 5% sheep blood) and TSB (Trypticase soy broth) supplemented with 10% glycerol and 5% lysed sheep blood. The sheep's blood and cysteine chloride were added to optimize bacterial growth.
| Product | Manufacturer | Ingredients |
|---|---|---|
| FluoroDose | Centrix, USA | 5% sodium fluoride |
| Polimo | Imicryl, Turkey | 5% sodium fluoride, xylitol |
| MI Varnish | GC Corporation, Japan | 1 - 8% sodium fluoride, 1 - 5% CPP-ACP, 30 - 50% polyvinyl acetate, 10 - 30% hydrogenated rosin, 20 - 30% ethanol, 1 - 5% silicon dioxide |
Abbreviation: CPP-ACP, calcium phosphopeptide-amorphous calcium phosphate.
In order to regenerate the bacteria, a uniform suspension was prepared by mixing one milliliter of MRS and TSB broth with the lyophilized form of L.A. and S.M., respectively. It was then incubated at 37°C for 48 hours. L.A. was cultivated using MRS agar and an anaerobic incubator of whitley jar gassing system. S.M. was grown using blood agar and a 10% CO2 incubator (manufactured by Binder Inc., Germany). Subsequently, microbiological tests were performed to confirm these strains (18). The bacteria were then stored in a freezer at -20°C to be used for testing. For microbiological studies, the stored bacteria were recultured and incubated under the above-mentioned conditions. When the bacterial colony was observed, microbial suspensions with 0.5 McFarland turbidity [comparable equivalent to 1.5 × 108 colony forming units (CFU/mL)] were prepared using the sterile physiological serum.
In the next step, we prepared different concentrations of fluoride varnishes. The varnishes were first dissolved in 40% dimethyl sulfoxide (DMSO). Then, with distilled water, we applied the DMSO volume to the varnish volume to provide the varnish with a concentration of 100%. Subsequently, by adding water and DMSO mixture to this solution, concentrations of 50, 25, 12.5, and 6.25% of each varnish were prepared. The antibiotics of erythromycin and ampicillin were used for quality control of the test. These antibiotics were prepared according to the manufacturer's instructions (Sigma-Aldrich Chemie GmbH, Germany) with concentrations of 128, 64, 32, 16, 8, 4, 2, 1, and 0.5 µg/mL.
We evaluated the antimicrobial activity of fluoride varnishes using the disk-diffusion agar method, according to the standards of Clinical and Laboratory Standards Institute (CLSI) (19). The strains of S.M. and L.A. were cultured on MRS agar and Müller blood agar, respectively, through a flocked swab as lawn culture method. Six millimeters dry filter paper discs (Padtan Teb Co.) were then placed on the agar plates, and 20 microliters of different concentrations of fluoride varnish solutions were added to them. In this experiment, in addition to erythromycin and ampicillin solution, erythromycin 15 µg disk and ampicillin 10 µg disk (Padtan Teb Co.) were also considered positive control. A mixture of DMSO and distilled water was used for negative control. Also, in both culture media, a pure DMSO disk was considered to detect its antibacterial effect. Cultivated plates containing varnish and control discs were incubated at anaerobic (for L.A.) and a 10% CO2 (for S.M.) condition at 37°C. After 48 hours, the diameter of the inhibition zone (no bacterial growth) was measured in millimeters with a ruler. Each experiment was repeated five times for each bacterium, and an average of five measurements was counted as non-growth zone diameter. The diameter of the non-growth zone around varnish discs was compared with the used antibiotics and with each other. Using SPSS 16 software, the quantitative data were described as mean and standard deviation. Data distribution was normal. Analysis of variance (ANOVA) and post hoc tests were applied to compare the mean of non-growth zone diameter in the studied groups. The statistically significant level was considered P-value < 0.05.
4. Results
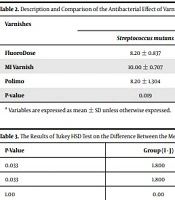
The antibacterial effects of varnishes, based on the diameter of the non-growth zone, are presented in Table 2. Separately, in each of the two groups of bacteria, S.M. and L.A., the highest diameter of the non-growth zone was observed due to MI varnish. In the next rank, Polimo and FluoroDose varnishes had similar diameters in terms of the non-growth zone. In the S.M. group, the severity of antibacterial effects of these three varnishes was statistically different (P-value = 0.019). The results of the post hoc test (Tukey HSD) showed that the mean difference of diameter of the non-growth zone between MI varnish and each of the Polimo and FluoroDose varnishes was statistically significant (Table 3). In the L.A. group, the severity of the antibacterial effect of varnishes did not differ significantly (P-value = 0.753).
| Varnishes | Diameter of Non-growth Zone (mm) | ||
|---|---|---|---|
| Streptococcus mutans | Lactobacillus acidophilus | P-Value | |
| FluoroDose | 8.20 ± 0.837 | 8.80 ± 0.837 | < 0.05 |
| MI Varnish | 10.00 ± 0.707 | 9.40 ± 2.302 | < 0.05 |
| Polimo | 8.20 ± 1.304 | 8.80 ± 0.447 | < 0.05 |
| P-value | 0.019 | 0.753 | |
a Variables are expressed as mean ± SD unless otherwise expressed.
| Bacteria | Group (I) | Group (J) | Group (I - J) | P-Value |
|---|---|---|---|---|
| Streptococcus mutans | MI varnish | Polimo | 1.800 | 0.033 |
| FluoroDose | 1.800 | 0.033 | ||
| Polimo | FluoroDose | 0.00 | 1.00 |
In intergroup comparisons, the average diameter of the non-growth zone caused by MI varnish, was higher in S.M. group than that of L.A group. The antibacterial effects of Polimo and FluoroDose varnishes on L.A. were greater than those of S.M. No bacterial growth halo was seen around the DMSO disc, and therefore this substance had no antibacterial effect.
5. Discussion
In this in vitro study, for the first time, the antibacterial effects of common sodium fluoride varnishes on both strains of S.M. and L.A. were measured and compared through disk diffusion method. In addition, the antibacterial properties of CPP-ACP and xylitol were compared. The antibacterial effect of each varnish was optimal against both bacteria. In both groups of bacteria, the highest antibacterial intensity was observed in MI varnish (containing CPP-ACP), followed by Polimo (containing xylitol) and FluoroDose varnishes with equal effect. In the disk diffusion method, the antibacterial evaluation of a chemical substance is influenced by the following factors: (1) the type of microorganism; (2) its sensitivity to the substance; (3) method of inoculation; (4) how the material spreads in the culture medium; and (5) chemical composition of that substance (4). MI varnish contains CPP-ACP. CPP is a milk-extracted protein that binds to calcium and phosphate ions to stabilize them as ACP. CPP-ACP attaches to plaque, hydroxyapatite, and soft tissue inside the mouth. It provides the bioavailable calcium and phosphate ions into saliva and plaque fluid and thus stimulates remineralization (20). In vitro studies have shown that when CPP-ACP is placed on the tooth surface, it can produce subsurface mineral products through interaction with hydrogen ions and diffusion into the enamel (21). In an in vitro pH-cycling study, which was designed by Ogata and et al. for 4 days on 28 bovine enamel slabs, it was demonstrated tissue loss on the enamel surface in the groups treated only with sodium fluoride (NaF) solution. The demineralization was reduced, and the enamel surface was protected with a mixture of the NaF solution and CPP-ACP paste (22). It is also reported that CPP-ACP has buffering and antibacterial effects on the plaque and interfere with the growth and adhesion of S.M. and S. sorbinus (23). CPP-ACP has been shown to significantly reduce decay activity in a dose-dependent manner. One percent CPP-ACP resulted in 55 and 46% decrease in smooth surface caries and fissure caries activities, respectively; similar to the effect of 500 ppm fluoride (24). In this study, MI varnish showed the highest antibacterial effect against S.M. and L.A. compared to the other two varnishes. It appears to be due to the bactericidal or bacteriostatic properties of high concentrations of free extracellular calcium (25). In this experiment, growth inhibition of S.M. and L.A. by Polimo varnish was observed, which can be attributed to the presence of xylitol. Laboratory and clinical studies have shown that xylitol is an anti-cariogenic agent that inhibits the growth and metabolism of S-mutant bacteria and reduces their count in plaque and saliva (26-28). These effects can result from several mechanisms, the xylitol-5-phosphate metabolite inhibits microorganisms’ growth and acid production by disrupting energy production processes within S.M. cells. The oral bacteria are incapable to ferment xylitol so that falling of plaque pH is prevented. Xylitol increases ammonia and amino acid concentrations in plaque, thus neutralizing plaque acidic conditions. Finally, xylitol-resistant S.M. strains are less virulent in the dental environment (29). Although S.M. is the target organism of xylitol, only some specific strains are inhibited. Furthermore, the inhibition intensity varies between different strains (30). Some other bacterial species are also susceptible. Previous researches have indicated that xylitol inhibits Lactococcus lactis at high concentrations over time, but not S. salivarius and L. casei (29). In the present study, growth inhibition of L.A. by Polimo varnish containing xylitol is remarkable. In addition, xylitol reduces extracellular polysaccharides and lipoteichoic acids by blocking the bacterial glycosyl transferase enzyme, thereby reducing the adhesion of biofilm to the tooth surface (31). Fluoride ions in low concentrations also inhibit the production of this enzyme. In 2005, Maehara and colleagues reported the synergistic inhibitory effect of xylitol and fluoride on the acidic production in S.M. in a laboratory study. Mediator analysis showed that xylitol and fluoride blocked the initial and final stages of intracellular glycolytic pathway, respectively (32). In 2018, Jafari et al. studied the antibacterial effects of four common varnishes, including Polimo and V-varnish (both containing xylitol), MI varnish (containing CPP-ACP), and Preventa against S.M. (33). Contrary to our results, Polimo varnish had the highest antibacterial effect, and MI varnish had the most effect. No growth inhibition was observed in V-varnish and Preventa. In both studies, disc diffusion method was used, but in Jafari et al. study, the varnishes were tested pure and with the same form that could be set with the teeth, so antibacterial effects were not very clear. We solved varnishes in the DMSO, which reduced the resin and adhesion properties, and their antibacterial effect was better identified. Although the caries-preventive and cariostatic effects of xylitol have been demonstrated, its antagonistic effects on dental caries are still debatable (34). Numerous in vivo trials have provided evidence about the effectiveness of xylitol in reducing S.M. counts in plaque and saliva, but one of the latest systematic reviews has concluded that these pieces of evidence are insufficient and have poor quality (35). In our investigation, the antibacterial effect of FluoroDose varnish, which lacks xylitol or CPP-ACP, was similar to that of Polimo varnish. The reason for this finding seems to be a difference in the varnish construction formulation. Previous researches, including that of Shen et al., have shown that in addition to dosage, the difference in carriers and the intensity of uniformity affect the maintenance and release behavior of different fluoride varnishes. They said that the fluoride content could vary even between doses taken from the same tube, and the uneven appearance of the varnish coming out of the tube indicates heterogeneity in the ingredients, and the result is a variety of fluoride content of different varnishes. This affects the severity of the antibacterial effect (17).
This in vitro study had some limitations, including low number evaluations with short intervals and only with a few intended concentrations of varnishes without considering environmental factors for measuring their antibacterial effects.
Similar in vivo studies on saliva and dental plaque are suggested to shed more light on the mechanism and severity of the antibacterial effect of xylitol and CPP-ACP in varnishes. It is also recommended to consider natural oral environment factors, including the quality of saliva, saliva pH, and other oral microbial flora through clinical studies. In all of these studies, it would be appropriate to measure the antibacterial effect of varnishes in more frequent time points and longer periods, such as ≥ 4 hours, and quantifying the released fluoride on different time points.
5.1. Conclusion
Fluoride varnishes of MI (containing CPP-ACP), Polimo (containing xylitol), and FluoroDose, under in vitro conditions, inhibit the growth of Streptococcus mutans and L.A. bacteria with good favorable intensity. The use of these varnishes seems to be suitable for preventing tooth decay. MI varnish is preferable because of its higher antibacterial properties.