1. Background
Morquio syndrome, mucopolysaccharidosis type 4 (MPS IV), is an autosomal recessive lysosomal storage disease in which the body cannot process certain glycosaminoglycans (GAGs). In MPS IV, the defect of the N-acetyl-galactosamine-6-sulfatase enzyme leads to accumulation of GAG chondroitin-6-sulfate (C6S) and keratan sulfate (KS), which are synthesized primarily in the cartilage. This causes various skeletal manifestations, multisystemic impairments, and significant morbidities (1, 2). The estimated rate of MPS IV is approximately 1 in every 71,000 live births in the United Arab Emirates, 1 in every 500,000 live births in Japan (3), and 1 in every 200,000 live births in Australia (4). Prenatal diagnosis is possible through enzyme detection in chronic villous samples (5). Postnatal diagnosis is performed through enzyme detection in plasma and fibroblasts and the presence of KS in urine (4).
Patients with MPS IV usually appear normal at birth. Distinctive skeletal abnormalities often appear in the first years of life and include disproportionate short stature with shortness of trunk and neck, pectus carinatum, kyphoscoliosis, genu valgum, joint laxity with gradual loss of ability, wrist hyperextension, and spinal complications such as odontoid hypoplasia, cervical instability and kyphoscoliosis (2, 6, 7), which may result in frequent surgical interventions (1, 8). The severe spinal abnormalities associated with MPS IV may contribute to cardiovascular and respiratory problems such as obstructive sleep apnea (OSA). OSA commonly occurs and is caused by an enlarged tongue and hypertrophy of the adenoids, tonsils, and vocal cords (9). Tracheal complications are caused by an imbalance in the growth of the trachea, innominate artery, spinal cord, and chest wall (10). Cardiovascular diseases, such as coronary heart disease, mild mitral or aortic valve thickening, pulmonary or systemic hypertension, and ischaemic heart disease, have been reported (11). Frequent otitis media and inner ear anomalies may lead to progressive sensorineural or conductive hearing loss starting at the end of the first decade of life (8, 12). Ophthalmological problems include pseudoexophthalmos secondary to shallow orbits, retinopathy, and optic neuropathy, with mild corneal clouding and opacification that worsens with age (1, 8). Other signs include a wide mouth, small hypoplastic teeth, tooth decay, thin enamel, inguinal hernia, and coarse facies, although IQ is within the normal range (8).
Elosulfase alfa (VimizimTM) was introduced as the first medication approved by the US Food and Drug Administration (FDA) for the treatment of MPS IV in April 2014 (1, 2, 13, 14) and by National Institute for Health and Care Excellence (NICE) in the UK in December 2015 (15). Elosulfase alfa is an enzyme replacement therapy (ERT) providing exogenous N-acetylgalactosamine-6-sulfatase, which improves the catabolism of C6S and KS (15). Several studies have shown favorable safety and efficacy results for treating patients with MPS IV using elosulfase alfa (2, 16-18). A double-blind, placebo-controlled study in 2014 showed significant improvement in primary activities and substantial reduction of urine KS (uKS) in MPS IV children older than five years of age (16). Another open-label study in 2015 was designed to explore the safety and efficacy of elosulfase alfa in MPS IV patients younger than five years of age (17).
In 2014, the use of elosulfase alfa was approved in the European Union, Canada, the United States, Australia, and Brazil to treat Morquio A syndrome. Elosulfase alfa is administered intravenously once-weekly at a dose of 2.0 mg/kg (18). There is no consensus in reports regarding the beneficial effects of using elosulfase alfa on patients with Morquio A syndrome.
The primary aim of previous research was to assess the safety and efficacy of elosulfase alfa on pediatric patients based on adverse event reporting and increase in physical endurance, respectively. Quality of life, respiratory function, and cardiac measures have also been explored in controlled studies (16, 17).
2. Objectives
Our study aimed to compare the impact of treatment with elosulfase alfa on pediatric patients in a real-world clinical setting with results from previously reported clinical trials, focusing on growth in height and weight, physical function, cardiac and respiratory outcomes, and pediatric quality of life.
3. Methods
3.1. Study Design
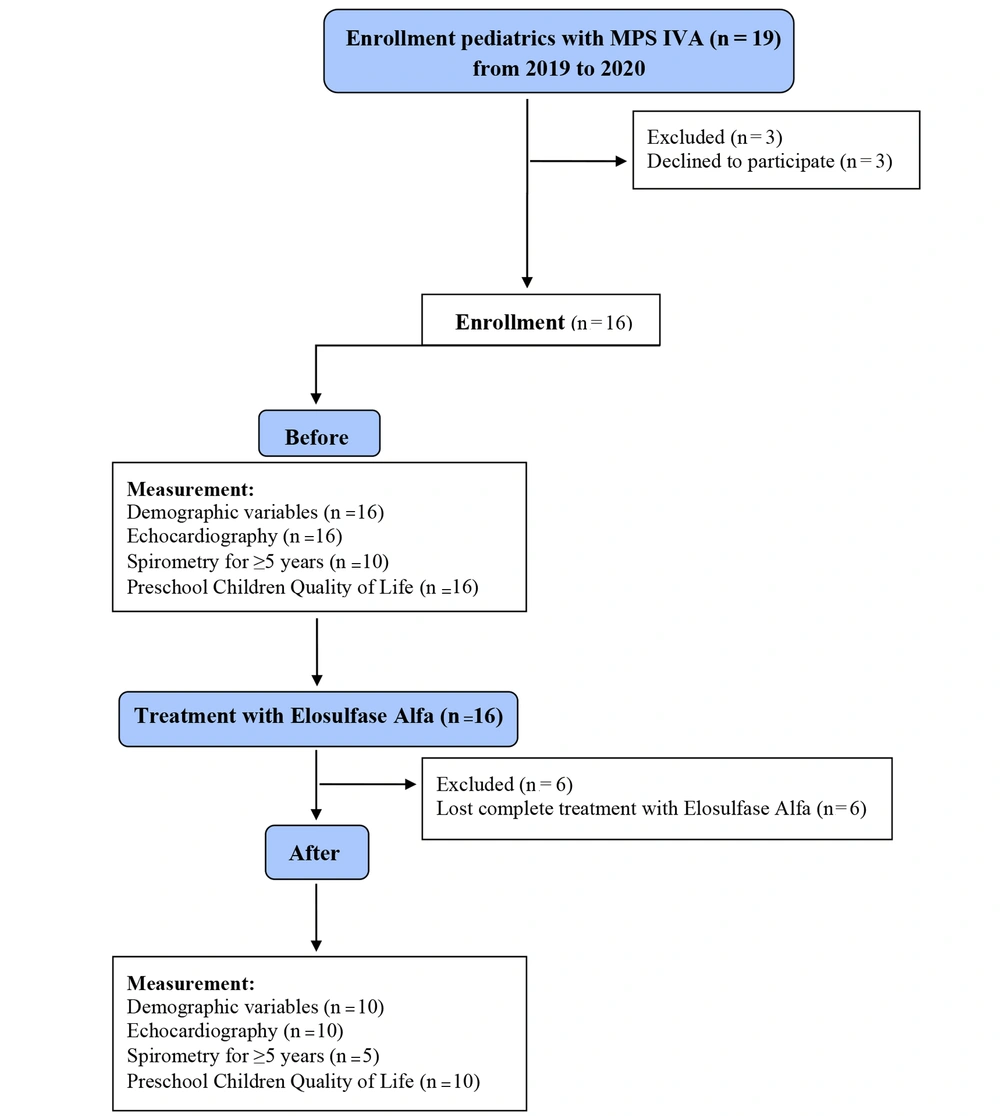
This before and after study was a clinical trial among pediatrics with MPS IV referred to the metabolic clinic of the Mofid Children’s Hospital, Tehran, Iran, from April 2019 to May 2020. We enrolled all pediatricians with MPS IV via simple sampling. Some patients declined to participate mainly due to incomplete treatment duration, and ten pediatricians with MPS IV were enrolled. Figure 1 shows the sampling flowchart.
3.2. Interventions and Measurement
Elosulfase alfa was used to treat MPS IV with the standard dose of 2 mg/kg/weekly, every Sunday at 5 pm, through intravenous (IV) infusion for 54 weeks, concerning the patients’ general conditions.
Data collection was performed by a trained research assistant, a subspecialist in pediatric endocrinology, before the first time and after the last time of prescription using a pre-designed checklist. Data collection was done based on a physical exam by the attending pediatric endocrinologist, a paraclinical report, and parents' pediatrics.
3.3. Measurement
The measurement checklist included demographic variables, physical examination, biometric variables, and laboratory and paraclinical data. Also, the pediatric quality of life was measured using the TNO-AZL Preschool Children Quality of Life (TAPQOL) questionnaire distributed among the patients’ mothers. The variables were assessed before and after prescription, and the correlations between the variables and elosulfase alfa prescription were analyzed and compared.
Demographic variables, including sex, age, height, and weight, were performed in a metabolic clinic by a constant person, using a fixed method (standing height beside a mechanical wall mount stadiometer and weight measuring with a Seca digital weighing scale) by the fellowship of a pediatric endocrinologist.
Physical examinations for physical ability assessment, including 6 minutes' Walk Test (6MWT), duration (second) of passing 6 meters distance, duration of standing on one leg, and duration of stair climb test, were performed for each case patiently and carefully by the fellowship of a pediatric endocrinologist.
In the second step, the patients were admitted to the Children's Cardiology Clinic, and echocardiography was carefully performed by a pediatric echocardiologist. The participants aged five years and more were also admitted to the Spirometry Ward of the Mofid Children Hospital. The results of spirometry were reviewed and analyzed accurately by the same expert clinician and were recorded meticulously. The patients underwent spirometry assessments including forced vital capacity (FVC), forced expiratory volume (FEV1), and forced expiratory flow at 25 - 75% of FVC (FEF25 - 75%). For the evaluation of biometric variables, the cardiac and pulmonary parameters for each patient were recorded using pediatric echocardiologist and spirometry reports, respectively.
TAPQOL, a preschool children quality of life questionnaire, examines the QOL construct and includes 43 items and an individual characteristic with four subgroups (physical, social, cognitive, and emotional). The mothers completed the questionnaire in a quiet room in 15 - 20 minutes. TAPQOL has different scoring methods according to the question types (three- and four-point Likert scale), with higher scores indicating a better QOL (19). The psychometric study of the Persian version of this tool among Iranian adolescents reported a reliability of 66.0-88.0, which is acceptable (20).
3.4. Data Analysis
Data analysis was performed in SPSS version 22 (SPSS Inc., Chicago, IL, USA). The quantitative variables were expressed as mean and standard deviation (SD). Pearson’s correlation coefficient and the comparisons of different variables and the Wilcoxon signed ranks test were used to evaluate differences between variables. A P-value less than 0.05 was considered statistically significant.
3.5. Ethics Approval and Consent to Participate
The present study was extracted from a thesis for a subspecialist degree in pediatric endocrinology, submitted to the Shahid Beheshti University of Medical Sciences, Tehran, Iran. All study ethical considerations were registered and approved by the institutional review board and the research ethics committee of the Shahid Beheshti University of Medical Sciences (Ethical Code: IR.SBMU.MSP.REC.1396.662). The parents of pediatricians with MPS IV were informed of the study objectives and signed a written informed consent form. They were assured of the confidentiality of personal information and the voluntary nature of participation.
4. Results
Ten pediatricians with MPS IV were included in our study, and seven (70%) of them were female. The minimum, maximum, and mean ± SD ages of the pediatricians were 3, 10, and 5.8 ± 2.3 years, respectively.
Seven parents of the patients presented mutation analysis that was quested by their familial gynecologist for healthy fertility. Table 1 shows the mutation analysis for seven pediatricians with MPS IVA. Genomic analysis results revealed two novel mutations, homozygous c.1002+1G>A and c.609delC (p. [Asn204ThrfsTer115]).
| Patient | Gender | Enzyme Activity | First Allele | Second Allele | Reference |
|---|---|---|---|---|---|
| 1 | M | 0.0 | c.688T>C | p.Trp230ARg | Tomatsu et al. 1995 (21) |
| 2 | M | 0.1 | c.1480A>G | p.Met494Val | Bunge et al. 1997 (22) |
| 3 | F | 0.0 | c.346>A | p.Gly116Ser | Tomatsu et al. 2004 (23) |
| 4 | F | 0.1 | c.949G>C | p.Gly317Arg | Bidchol et al. 2014 (24) |
| 5 | M | 0.1 | c.346G>A | p.Gly116Ser | Tomatsu et al. 2005 (25) |
| 6 | F | 0.0 | c.1002+1G>A | c.1002+1G>A | Novel |
| 7 | M | Unavailable | c.[609delC];[609delC] | (p.[Asn204ThrfsTer115];[Asn204ThrfsTer115]) | Novel |
The results showed, before Elosulfase alfa prescription, the minimum, maximum, and mean ± SD of height and weight of the pediatricians were 81, 93.5, 89.6 ± 3.7 cm, and 11, 16, 14 ± 1.5 kg, respectively. After treatment by Elosulfase alfa, the minimum, maximum, and mean ± SD of height and weight of the pediatricians became 88.5, 96, 93.6.6 ± 2.6 cm, and 12, 17, 15.3 ± 1.5 kg, respectively.
Although the results showed that the mean height and weight improved after treatment by Elosulfase alfa, there was no statistically significant difference between height and weight before and after the study.
The mean time ofphysical ability variables improved. The mean change from baseline for time to walk 6 m was 3.88 ± 6.14 seconds with a range of 0.76 to 20.86 (P = 0.005). The mean change from baseline in time for standing on one leg was 4.54 ± 6.59 seconds with a range of 0.51 to 21.58 (P = 0.005). The mean change from baseline for the stair climb test was -20.12 ± 17.44 seconds with a range of -58.58 to 2.72 (P = 0.007). The mean change from baseline for meters walked in the 6MWT was 45.05 ± 54.56 meters with a range of 62 to 131.5. (P = 0.005). The mean change from baseline on the QOL measure was 27.8 ± 22.98, with a range of 8 to 61 (P = 0.015). Statistically significant differences were seen versus baseline across all physical assessments and the QOL measure. Pulmonary and tricuspid valves had no pathologies before or after ERT. Pulmonary artery pressure was normal in all the cases during the study. Four patients left ventricular hypertrophy (LVH) at the beginning of the study, and six were normal. No changes occurred after treatment by Elosulfase alfa, and no statistically significant changes were seen versus baseline in cardiac measures (Table 2).
| Assessment | Pre-treatment; Mean ± SD | Post-treatment; Mean ± SD | P-Value a |
|---|---|---|---|
| Demographic | |||
| Z-Score for height | -4.83 ± 2.2 | -4.88 ± 1.98 | 0.799 |
| Z-Score for weight | -3.18 ± 2.02 | -3.3 ± 1.87 | 0.221 |
| Walk seconds of 6 m walking | 13.03 ± 11.19 | 9.15 ± 5.19 | 0.005 |
| Physical Ability | |||
| Stand seconds of on one leg | 3.37 ± 2.52 | 7.91 ± 7.74 | 0.005 |
| Stair seconds of climb test | 38.41 ± 23.22 | 26.21 ± 9.97 | 0.007 |
| Seconds of 6 minutes' walk test | 261.85 ± 74.86 | 306.90 ± 72.76 | 0.005 |
| Echocardiography (%) | |||
| Ejection fraction (EF) | 61 ± 5.83 | 61.70 ± 4.14 | 0.241 |
| Systolic fraction | 30.7 ± 3.68 | 30.3 ± 3.83 | 0.234 |
| Forced expiratory volume | 85.2 ± 27.6 | 57.4 ± 8.1 | * |
| Forced vital capacity | 81.7 ± 26.6 | 45.2 ± 6.6 | * |
| Spirometry (%) | |||
| Forced expiratory flow | 71.1 ± 28.1 | 84.2 ± 19.5 | * |
| Delta forced expiratory flow 1 after bronchodilator | 5 ± 0.4 | 14.2± 12.3 | * |
| Quality of Life | |||
| Score of TAPQOL | 87.5 ± 25.4 | 115.30 ± 10.35 | 0.004 |
a *Five pediatricians with MPS IVA were less than five years, and spirometry was done for five pediatrics.
Echocardiography was performed for every patient before and after treatment by elosulfase alfa. At the beginning of the study, seven cases had mild mitral valve regurgitation, and three cases were normal. Control echocardiographs showed no improvement or reduction of valvular involvement. Among the patients, one had mild and one had moderate aortic valve regurgitation and insufficiency (AR/AI). No change was reported in the left ventricular (LV) Tei index, atrioventricular (AV) size, right ventricular (RV) Tei index, tricuspid annular plane systolic excursion (TAPSE), mitral annular plane systolic excursion (MAPSE), and E/A ratio (Table 2).
In our research, six pediatricswere five years old and more who were referred for spirometry, and one could not complete maneuvers required to complete the assessment. Thus, five pediatricians with MPS IVA underwent spirometric assessment. The remainder of the patients underwent pre- and post-post treatment bronchodilator spirometry (Table 2).
Eight pediatricians with MPS IV had sinusitis at the beginning of the study; they had facial pain, a "stuffed-up" nose, and a loss of smell. After treatment by Elosulfase alfa, none of them had sinusitis.
5. Discussion
A key goal of treatment with Elosulfase alfa is to improve physical function (16, 17, 26) and quality of life based on the four standard subgroups (physical, social, cognitive, and emotional) for MPS IV patients. Patients in our study showed statistically significant improvement in physical abilities and QOL measures over 54 weeks of treatment with elosulfase alfa.
The results of stair climbing and walk tests are consistent with reports from clinical trial reports, although they used some different assessments. Improvements in 6MWT are more pronounced in our study than in Hendriksz et al.’s study, potentially due to the longer length of treatment (54 versus 24 weeks) in our study (16). No significant improvement in 6MWT results was reported by Musson et al., and Burton et al, which were also weeks of treatment (27, 28). This may be due to their shorter time frame compared to our study or the different age spectrum of their patients (> 7 years old) (16, 28). Burton et al. (28) reported improvements in stair climbing ability, consistent with our findings, while subjects in Hendriksz et al. (2015) showed no improvement in the 3-minute stair climb (3MSC) after ERT (17). Differences may be due to different assessments and length of treatment.
One of the most important parts of this research was studying growth measurements: weight and height. In contrast to Jones et al. (2015) (26). We found no statistically significant difference in mean weight or height z-scores. This may be due to different study designs, as theirs was a controlled study, or their results were potentially due to the deteriorating nature of MPS IV (1). On the other hand, since MPS IV patients often experience significant deteriorations in height and weight in the absence of ERT, reaching a plateau or having smaller decreases in height and weight on growth charts are both favorable outcomes.
Treatment with elosulfase alfa has positively impacted psychological and quality of life outcomes, assessed using the TAPQOL in four main standard fields (physical, social, cognitive, and emotional). The mean score was 87.5 at the beginning and 115.3 after one year of ERT. This improvement in QOL is consistent with other studies using different QOL measures and differing lengths of treatment. Hendriksz and colleagues in 2018 (29) and Hendriksz in 2016 (30) found that MPS-HAQ score improvements were greater for those with sustained treatment (96 weeks) with elosulfase alfa. Lavery and colleagues also reported QOL score improvement in 6% of cases and stability in 91% of 35 MPS IV patients using MPS-HAQ (31). While MPS-HAQ is validated for patients with MPS, we used TAPQOL as it is appropriate for the age of our patients. Some variation in results may be directly related to different assessment tools, the cultures of patients in each study, and the heterogeneity of self-management among individuals with MPS IV (32).
Limitations of our study include a small sample size, which can be expected in studies of MPS IV because it is a rare disease. Some of our assessments could not be performed by patients < 5 years of age, which may have impacted the significance of results, especially respiratory assessments. Long-term studies are needed to further assess the impact of elosulfase alfa on growth, cardiac and respiratory manifestations, physical function, and quality of life in MPS IV to expand the knowledge and expectations of treatment and determine the minimum safe age for initiating treatment with elosulfase alfa.
5.1. Conclusion
We concluded that treatment with elosulfase alfa resulted in a significant improvement in quality of life and physical abilities, including walking 6 m, standing on one leg, stair climbing, after a mean follow-up period of 54 weeks in Iranian pediatrics.