1. Background
Recurrent respiratory tract infections (RRTIs) in China are defined by considering not only numbers but also ages (see Supplementary File) (1). Despite the introduction of new antibiotics and vaccines that have helped reduce the mortality and morbidity of RRTIs, these infections remain widespread and have an incidence of 20% that seems to be on increase in China (2, 3). Besides, RRTIs may cause a variety of complications that seriously affect the health and growth of children. Furthermore, RRTIs present significant problems for the families of these children, as they not only increase the treatment and hospitalization costs, but also result in a loss of working days for parents and caregivers (4). These adverse effects are multiplied in the case of recurrent infection (5), and RRTIs have, therefore, become a hot topic for pediatricians.
Mycoplasma pneumoniae (MP) is an important cause of upper and lower respiratory tract infections (6), and is responsible for 10 - 40% of cases of community-acquired pneumonia in pediatric patients (7, 8). Mycoplasma pneumoniae pneumonia (MPP) is usually a benign self-limiting disease. However, it may sometimes cause various extrapulmonary complications and, thereby, clinical symptoms last for several weeks due to the spread of infection or autoimmune mechanisms (9, 10). A recent review article reported that severe CAP caused by intracellular pathogens, including MP, accounts for approximately 1 to 7% of cases (11). With regard to its association with RRTIs, Principi et al. (12) argued that MP and Chlamydia pneumoniae play a more important role in causing respiratory tract infections than previously thought, and a major risk factor for identifying their presence is a history of recurrent respiratory infections. Stelmach et al. (13) analyzed 6,335 cases of children with RRTIs and found that 26.9% of them had persistent MP infection. Esposito et al. (14) also implicated MP as a causative pathogen for RRTIs in children, and reported that prolonged azithromycin therapy can significantly reduce the risk of recurrences. However, relatively few studies have reported the factors associated with RRTIs in the one-year period after clinical treatment of MPP in infants. Currently, there is no known specific treatment for children suffering from respiratory tract infections without immunological disorders (15). Therefore, it is essential for pediatricians to early diagnose respiratory tract infections, initiate prompt treatment, and prevent the progression of the disease.
In this study, by analyzing relevant clinical data on the occurrence and treatment of MPP in infants, the follow-up findings, and the occurrence of RRTIs within one year after MPP treatment (multimodality regimens including inhalation, azithromycin, and/or oxygen therapy), the body of the literature of RRTIs and MMP has been attempted to expand. Further, the factors associated with RRTIs after the occurrence of MPP were discussed, so as to aid the prognosis, prevention, and treatment of RRTIs in the future.
2. Methods
2.1. Study Population
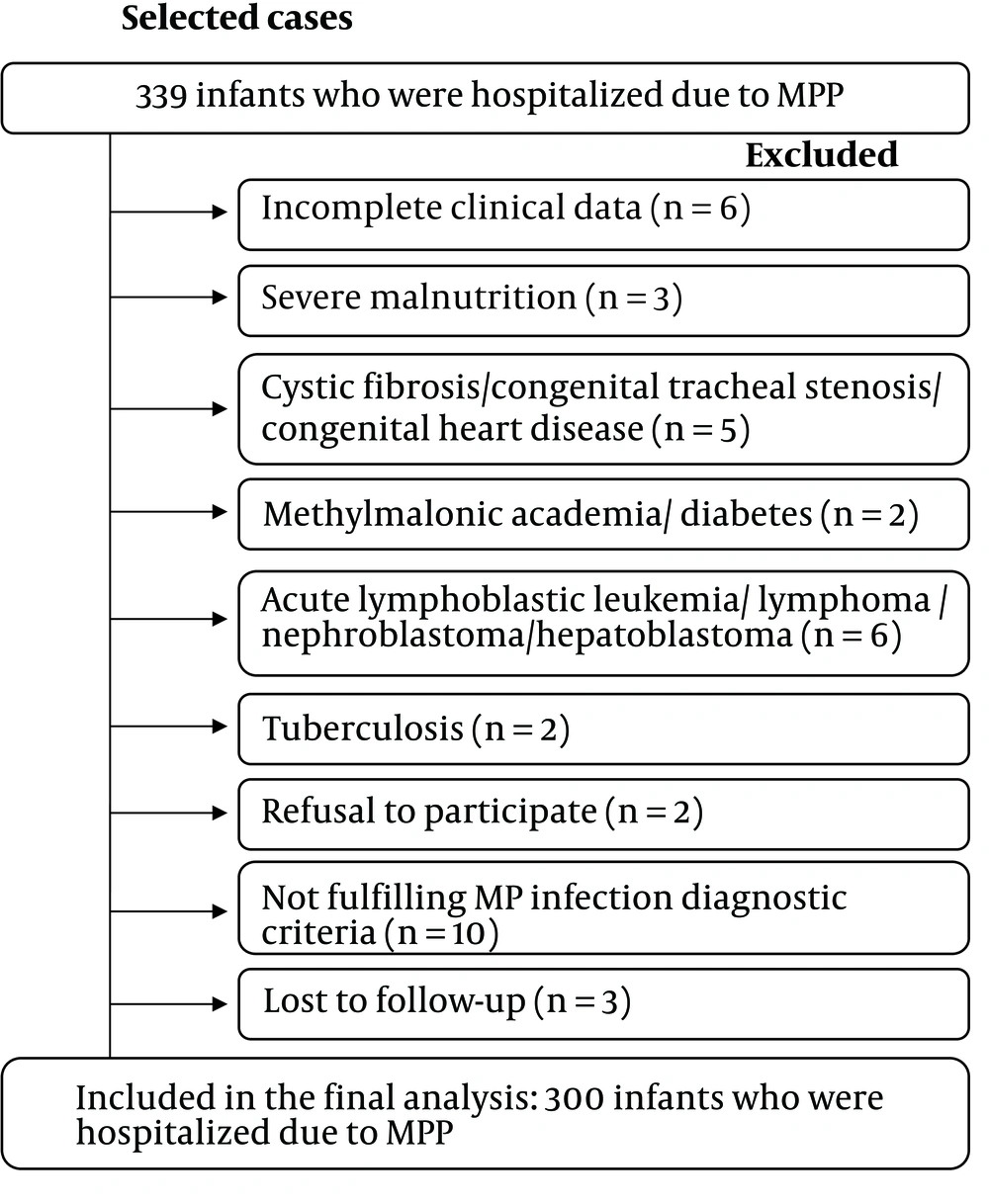
Between January 2015 and December 2018, infants diagnosed with MPP admitted to the 165-bed Pediatric ward of a tertiary hospital in Fujian Province, China, were enrolled in this study. Severe MPP or MPP was diagnosed according to standard guidelines (16) (see Supplementary File). For the diagnosis of RRTIs, the patient needs to meet at least one of the following criteria: No less than seven upper respiratory infections per year, no less than three episodes of tracheobronchitis per year, and no less than two episodes of pneumonia per year if the age of the patient is 0 - 2 years (1). The exclusion criteria were tuberculosis, a positive test for human immunodeficiency virus, malignancy, cystic fibrosis, congenital abnormalities of the respiratory tract such as lung and respiratory cilia, cyanotic heart disease, known metabolic disorders, diabetes, renal impairment, rheumatic diseases, immunosuppressive and steroid treatment, severe malnutrition, age over one year, and incomplete clinical data.
Written informed consent was obtained from at least one parent of each child prior to inclusion. The parents of the patients who were included participated in structured pediatrician-guided interviews that were designed to gather information about the patient and family medical history. Medical records containing the following data were obtained: (1) Age and gender of the patient, length of hospitalization, and duration of fever, (2) the results of ancillary investigations, including white blood cell (WBC) count, platelet (PLT) count, hemoglobin (Hb), C-reactive protein (CRP), lactate dehydrogenase (LDH), and D-dimer level, as well as chest radiography findings, (3) co-infections diagnosed using blood culture and deep sputum culture for bacterial infection (a positive response was defined as two consecutive positive tests for the same bacterial species), passive agglutination assay for MP antibody, indirect fluorescent antibody assay for nine common pathogens [respiratory syncytial virus, RSV; adenovirus, AD; influenza (INF) A and B viruses; parainfluenza viruses (PIVs) 1, 2, and 3; Legionella pneumophila, LP; Chlamydia pneumoniae, MP; and Coxiella burnetii, COX], fluorometric PCR test for the detection of Epstein-Barr (EB) virus and cytomegalovirus (CMV), colloidal gold assay for enterovirus type 71 (EV71), and enzyme-linked immunosorbent test for the herpes simplex virus in serum samples. Cases were reviewed in the first month following hospitalization, and at the same time, RRTI questionnaires were sent to the parents of MPP infants by WeChat. Questionnaires contained the number of upper respiratory infections, tracheobronchitis, and pneumonia, the titers and course of MP-IgG and positive IgM antibody, eczema, pet ownership, interior decoration, inhaled or ingested allergens, exposure to environmental tobacco smoke, and gastrointestinal function (including diarrhea, abdominal pain, abdominal distension, and vomiting). Exposure to environmental tobacco smoke was scored as follows: (1) 1 = occasional exposure outside the home; (2) 2 = regular exposure outside the home; (3) 3 = exposure to paternal or another adult smoking in the home; and (4) 5 = exposure to maternal smoking in the home. If there was more than one source of exposure, the points were added; that is, a child exposed to maternal smoking in the home and to a smoker who had frequent contact with the child but lived outside the home (e.g., a grandparent) would receive scores 5 and 2 that would be added to make up a final score of 7 (17). If the questionnaires were not returned within two weeks, a telephone interview was used. At the time of the review (an interval of 10 - 20 days or longer), blood samples were taken for serum MP antibody analysis. Follow-up investigations were conducted every three months with alternating clinic visits and WeChat until one year after discharge from the hospital.
2.2. Ethical Compliance
The research was authorized by the Ethics Committee of Fujian Maternity and Children Hospital, Affiliated Hospital of Fujian Medical University (No. 2017-042).
2.3. Statistical Analysis
All analyses were conducted using SPSS version 23.0 (IBM). The results of descriptive analyses are shown as frequencies or rates for categorical variables, medians (Q25-to-Q75 values) for continuous variables with non-normal distributions, and mean ± SD for continuous variables with normal distributions. Univariate differences among the study groups were compared by the χ2 test for categorical variables and one-way analysis of variance or Kruskal-Wallis test for continuous variables, as appropriate. Binary multivariate logistic regression modeling (dependent variable was RRTIs) was performed to determine factors associated with RRTIs. A P value below 0.05 was used to indicate statistical significance.
3. Results
3.1. Demographic Characteristics
Between January 2015 and December 2018, 339 infants with MPP were admitted. Based on the exclusion criteria, 300 infants with MPP were selected for the present research (Figure 1). This cohort was divided into two groups: the RRTI group (134/300, 44.7%) and the non-RRTI group (166/300, 55.3%). The two groups had a similar gender ratio (P > 0.05, Table 1). However, compared to the non-RRTI group, in the RRTI group, patients were younger, and a higher proportion of them had a history of prematurity [< 37 weeks gestational age (GA)] (P = 0.002 and 0.001, Table 1).
| Characteristics | Recurrent Respiratory Tract Infection Group (n = 134) | No recurrent Respiratory Tract Infection Group (n = 166) | F/Z/χ2 | P Value |
|---|---|---|---|---|
| Gender (males) | 83 (61.9) | 106 (63.9) | 0.117 | 0.733 |
| Age (mo) | 6.72 (3.95 - 9.57) | 7.93 (5.98 - 10.24) | -3.054 | 0.002 |
| Prematurity (< 37 weeks GA) | 20 (14.9) | 6 (3.6) | 11.984 | 0.001 |
| Length of fever > 7 d | 29 (21.6) | 39 (23.4) | 0.145 | 0.703 |
| Length of fever > 10 d | 19 (14.1) | 22 (13.2) | 0.054 | 0.816 |
| Extrapulmonary complications of two or more systems | 23 (17.2) | 17 (10.2) | 2.381 | 0.123 |
| Severe MPP | 53 (39.6) | 38 (22.9) | 9.739 | 0.002 |
| Length of hospitalization (d) | 8.57 ± 3.28 | 7.77 ± 4.14 | 3.341 | 0.069 |
| Co-infection | ||||
| Adenovirus | 3 (2.2) | 8 (4.8) | 1.398 | 0.237 |
| Cytomegalovirus | 43 (32.1) | 29 (17.5) | 8.353 | 0.004 |
| Epstein-Barr virus | 6 (4.8) | 9 (5.4) | 0.139 | 0.709 |
| Respiratory syncytial virus | 4 (3.0) | 6 (3.6) | 0.091 | 0.763 |
| Influenza viruses | 6 (4.8) | 4 (2.4) | 0.984 | 0.321 |
| Chlamydia pneumoniae | 19 (14.2) | 8 (4.8) | 7.931 | 0.005 |
| Co-infection with two or more pathogens | 20 (14.9) | 21 (12.7) | 0.325 | 0.569 |
| Laboratory examinations | ||||
| White blood cell count (× 109/L) | 14.43 ± 6.60 | 13.62 ± 7.01 | 1.050 | 0.306 |
| Hemoglobin (g/L) | 113.69 ± 17.27 | 109.45 ± 21.42 | 3.456 | 0.064 |
| Platelet count (× 109/L) | 381.07 ± 178.60 | 409.00 ± 198.35 | 1.606 | 0.206 |
| Absolute numbers of eosinophils | 0.28 (0.60 - 0.36) | 0.23 (0.40 - 0.31) | -1.767 | 0.077 |
| C-reactive protein (mg/L) | 31.71 (1.00 - 35.57) | 24.89 (0.68 - 24.64) | -1.415 | 0.157 |
| Lactate dehydrogenase (U/L) | 725.14 (323.65 - 846.50) | 786.75 (318.35 - 858.00) | -0.176 | 0.863 |
| D-dimer (mg/L) | 2.59 (0.46 - 4.37) | 4.03 (0.49 - 4.51) | -1.189 | 0.235 |
| Outcomes of follow-up | ||||
| Eczema | 49 (36.5) | 45 (27.1) | 3.083 | 0.089 |
| Pet ownership | 2 (1.5) | 5 (3.0) | 0.751 | 0.386 |
| Interior decoration | 22 (16.4) | 22 (13.3) | 0.593 | 0.441 |
| Inhaled or ingested allergens | 36 (26.9) | 21 (12.7) | 9.735 | 0.002 |
| Environmental tobacco smoke | 1.83 (0 - 5) | 1.75 (0 - 5) | -0.653 | 0.514 |
| Gastrointestinal function b | 42 (31.3) | 43 (25.9) | 1.080 | 0.299 |
Abbreviations: GA,gestational age; MPP, Mycoplasma pneumoniae pneumonia
a Values are expressed as No. (%) or mean ± SD unless otherwise indicated.
b Gastrointestinal function: appetite, nausea, vomiting, diarrhea, constipation, change in the character of stool (caliber, consistency, color), jaundice, dark urine, abdominal pain, hematemesis, melena, hematochezia, and heartburn.
3.2. Clinical Characteristics
Extrapulmonary complications were common among children with MPP, and the proportion of patients with extrapulmonary complications of two or more systems did not significantly differ between the two groups. Fever was the most common symptom in the whole cohort, and the duration of fever did not differ significantly between the two groups. However, the severity of the disease was a clinical concern, as the proportion of patients with severe MPP in the RRTI group was higher than that in the non-RRTI group. Despite this, the length of hospitalization did not significantly differ between the two groups. With regard to co-infection, cytomegalovirus was detected in 24.0% of blood samples (72 cases), and C. pneumoniae was detected in 9.0% of blood samples (27 cases). The proportion of patients with cytomegalovirus and C. pneumoniae co-infection in the RRTI group was higher than that in the non-RRTI group (32.1% compared with 17.5 and 14.2% compared with 4.8%, respectively). No differences were observed in the proportion of patients with adenovirus, Epstein-Barr virus, respiratory syncytial virus, mixed infection with influenza viruses, and co-infection with two or more pathogens (Table 1). In the one-year follow-up period after MPP, the proportion of patients with a history of exposure to inhaled or ingested allergens in the RRTI group was higher than that in the non-RRTI group. No differences were observed in the proportion of patients with eczema, pets, interior decoration, exposure to environmental tobacco smoke, and gastrointestinal function (Table 1).
3.3. Identification of Factors Associated with RRTIs
Binary logistic regression analysis was used to analyze the associations between statistically significant factors and RRTIs. The categorical variables analyzed were a history of prematurity, severe MPP, mixed infection with cytomegalovirus and C. pneumoniae, and a history of exposure to inhaled or ingested allergens. The Blom formula transformation was performed for the age data so that a normal distribution would be achieved. Thus, the independent variable that was eventually used in the analysis was the normal score of age as calculated using the Blom formula, a history of prematurity, severe MPP, mixed infection with cytomegalovirus, and C. pneumoniae, and a history of exposure to inhaled or ingested allergens. In the binary logistic regression analysis model, a history of prematurity (OR = 6.336, 95% CI: 2.337 - 17.116, P ≤ 0.001), a history of exposure to inhaled or ingested allergens (OR = 2.527, 95% CI: 1.289 - 4.956, P = 0.007), and C. pneumoniae co-infection (OR = 2.787, 95% CI: 1.145 - 6.784, P = 0.024) were found to be positively and significantly associated with RRTIs after clinical treatment of MPP, while age (OR = 0.894, 95% CI: 0.825 - 0.970, P = 0.007) was found to be negatively associated with RRTIs (Table 2).
| Variables | β | S.E | Wals | df | Sig. | Exp (β) | Exp (β) 95% Confidence Interval | |
|---|---|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | |||||||
| Age | -0.109 | 0.041 | 7.391 | 1 | 0.007 | 0.894 | 0.825 | 0.970 |
| Prematurity (< 37 weeks GA) | 1.846 | 0.509 | 13.167 | 1 | ≤ 0.001 | 6.336 | 2.337 | 17.116 |
| Inhaled or ingested allergens | 0.927 | 0.344 | 7.283 | 1 | 0.007 | 2.527 | 1.289 | 4.956 |
| Chlamydia pneumoniae | 1.025 | 0.454 | 5.099 | 1 | 0.024 | 2.787 | 1.145 | 6.784 |
Abbreviation: GA, gestational age.
4. Discussion
As known, RRTIs are not uncommon in infants in the first year of life after MPP is clinically cured, but the factors that are likely to cause RRTIs in such cases are unclear. This study investigated and analyzed these factors in order to present a list of potential predictors of RRTIs in infants who had been previously treated for MPP. Namely, age, a history of prematurity, previous exposure to allergens, and co-infection involving C. pneumoniae were identified as important indicators of the future occurrence of RRTIs.
This study showed that 44.7% of hospitalized infants acquired RRTIs after the end of the follow-up for clinically cured MPP. A previous study showed that among infants who required hospitalization due to viral LRTI, 30% - 50% acquired recurrent respiratory illnesses (18). The prevalence was similar in MP RRTIs and viral RRTIs. At present, the pathogenesis of pediatric RRTIs is not clear, although it has been reported that it may be associated with congenital factors (such as underlying respiratory or cardiovascular disease), immunosuppression, and deficiency of trace elements (19). Therefore, the exclusion of patients with underlying diseases can improve the identification of readmission risk factors associated with RRTIs in infants in their first year of life who have been clinically treated for MPP (20).
This study found younger infants had a higher increase in RRTIs after clinical treatment of MPP. This is probably attributable to the immaturity of the immune system of infants of this age and exposure to pathogens (21). The immune system has been shown to have a degree of immaturity from birth until six to seven years of age. This immaturity may be associated with age-related functional disorders in immune response (22). With regard to the second probable cause, MP may be an important pathogen in infants with lower respiratory tract infections in China (23). Colin et al. (24) reported that MP infection may precede and intensify subsequent infections with various respiratory viruses and bacteria. Further, MP infection may also cause increased immune exhaustion during infections (25). Together, these results indicate that RRTIs occur in infants of a younger age who have an immature immune system and are associated with the presence of concomitant infections.
This study also showed that a history of prematurity was associated with RRTIs within one year after clinically treated MPP. Due to the deficiency of both humoral and cellular immune host defenses, premature infants have been shown to have a mycoplasmal infection (26). The mycoplasmas may be responsible for chronic sinopulmonary disease in a majority of such patients (27). In agreement with our finding, a retrospective longitudinal cohort of young children (≤ 3 years) showed that during the first episode of viral lower respiratory tract infection (often termed viral bronchiolitis), the risk of recurrence within 12 months post-hospitalization was associated with a history of prematurity (17). Morata-Alba et al. (28) have reported that spirometric measurements at age 8 - 9 years were lower in children born at 33 - 34 weeks GA than in those born at term. Spirometric parameters (such as the Tiffeneau-Pinelli index and FEV1) are significantly correlated with obstruction. Thus, prematurity might also be associated with obstruction, and therefore, abnormalities in lung function, and this, in turn, may favor the occurrence of RRTIs. Additionally, during pulmonary infection with MP, auto- and/or paracrine mediators induce pathophysiological changes in the alveolar-capillary barrier, leading to the accumulation of edema and impaired alveolar fluid clearance (29). This mechanism may also favor the occurrence of RRTIs in first-year infants after MPP.
Atopy is described as symptomatic sensitization to one or more allergens; thus, an individual with confirmed allergic sensitization is clinically diagnosed with an allergy (30). Besides, MP infection has been shown to exacerbate many mechanisms associated with allergic inflammation, including T-helper type 2 responses and airway hyperreactivity (31). In fact, a nationwide cohort study reported that the underlying immune dysfunction and airway inflammation that characterizes atopy might contribute to MP infection in patients (32). Additionally, a previous study of the long-term impact of MP on allergic inflammation showed that airway inflammation remained unresolved for a considerable period of time, even after the resolution of pneumonia-like symptoms (33). Wang et al. (34) found that serum IL-17 levels in the recovery phase were significantly higher in atopic children with MPP than in non-atopic children with MPP and atopic children without MPP. This finding confirms that IL-17-dependent allergic inflammatory reactions are exacerbated by MP infection in atopic children. Similarly, our results showed that a history of exposure to inhaled or ingested allergens is a risk factor for RRTIs in infants in the first year of life after clinical treatment of MPP. Thus, MPP patients with a history of exposure to inhaled or ingested allergens may go through a prolonged period of chronic airway inflammation and airway hypersensitivity that may eventually result in RRTIs. Additionally, it has been reported that atopic patients have low levels of cytokines such as IFN-γ, which play an important role in the Th1 response and are important for the control of infections; this might explain why children with allergies are more susceptible to RRTIs (35).
Interestingly, our study showed that C. pneumoniae co-infection was detected in 9.0% of blood samples. A multicenter, prospective, epidemiologic cohort study initiated by the German Competence Network for Community-acquired Pneumonia indicated that C. pneumoniae was detected in 3.9% of swab samples (36). Another consecutive cross-sectional study showed that C. pneumoniae was detected in 10.52% of nasopharyngeal samples (37). The conflicting results might be due to differences in the study populations, types of specimens, and ways of measuring C. pneumoniae. Although MP and C. pneumoniae mix infection is not uncommon in the population, its clinical implications are not clear (38). Besides, MP and C. pneumoniae could contribute to recurrent wheezing and exacerbation of asthma (39). In our study, C. pneumoniae co-infection had a significant positive correlation with RRTIs in infants in their first year of life who had been clinically treated for MPP. Co-infection with MP and C. pneumoniae may be responsible for the activation of cellular elements in bronchial tissue that induces the release of a cytokine cascade and results in airway remodeling, along with the secretion of IL-8 and TNF, thus resulting in the severe bronchus and lung tissue damage (40), and potentially, RRTIs.
The limitations of this study are its retrospective design and small sample size. Despite these limitations, our results provide important insights into the factors associated with RRTIs within one year after clinical treatment of MPP in infants.
4.1. Conclusion
To conclude, RRTIs within the first year following clinically cured MPP in infants is relatively common (44.7% in this study) and is related to the patients’ age, history of prematurity, history of exposure to inhaled or ingested allergens, and co-infection with C. pneumoniae. Paying careful attention to these clinical variables would have important clinical implications, since early recognition and adequate management of those factors that are modifiable may reduce RRTIs morbidity. Next, additional prospective studies with large sample sizes are required.