1. Background
Trisomy 18 (Edwards syndrome, T18) is the second most common trisomy aneuploidy (1). This chromosomal anomaly is characterized by multiple congenital anomalies frequently involving cardiac, respiratory, neurological, gastrointestinal, genitourinary, and skeletal malformations (2). Congenital heart defect is detected in about 90% of patients with T18; particularly, ventricular septal defect (VSD), atrial septal defect (ASD), and patent ductus arteriosus (PDA) (3). Furthermore, more than 50% of T18 patients are clinically associated with craniofacial abnormalities such as prominent occiput, low-set and malformed auricles, narrow bifrontal diameter, and micrognathia. In general, 10% - 50% of T18 cases showed thorax, hand, or foot anomalies; pulmonary and cardiac stenosis; renal anomaly with horseshoe defect; polycystic kidney; hydronephrosis, or double ureter. In addition, more than 50% of T18 cases had central nervous system abnormalities such as cerebellar hypoplasia, meningomyelocele, agenesis of corpus callosum, Dandy-Walker malformation, and hydrocephalus (4). All cases had severe psychiatric and psychomotor disorders. Based on the reported literature, the prevalence of live birth in T18 cases ranges from 1/3000 to 1/10,000, with an estimated average of about 1 in 6,000 (5-8). Sex difference during pregnancy is identified with a 4-fold higher incidence rate in females compared to males (9).
Most population-based studies have reported that patients with trisomy 18 do not live beyond the first year of life, and the majority of articles reported a 1-year survival rate of 10% (1, 2, 10). Because of these poor outcomes, cardiac surgery has not been justified and is performed in a limited number of patients with T18. Provision of comfort and palliation without surgery or interventions had been accepted as the main treatment, even though patients with congenital heart disease (CHD) were expected to be normalized hemodynamically by cardiac surgery (11). However, improvements in surgical technique and post-operative care have led to more aggressive interventions for correction of CHD, even in patients with T18. Graham et al. (12) reported that complete repair in 35 patients with Trisomy 13 and T18 showed 90% survival at discharge. Additionally, in 2005, T18 was excluded from the list of conditions in which “resuscitation is not indicated” in the American Academy of Pediatrics neonatal resuscitation guidelines (13). After 2005, many reports showed improved outcomes after cardiac surgery in T18 patients (11, 14-17). However, many pediatricians and surgeons still hesitate to perform total cardiac repair under cardiopulmonary bypass, preferring palliative surgery without cardiopulmonary bypass because the post-operative course after palliative surgery has been generally accepted as less complicated. However, there are limited data about post-operative course and survival after palliative surgery in these patients (18, 19).
2. Objectives
We aimed to evaluate palliative cardiac surgery and compare it to total cardiac repair in patients with T18.
3. Methods
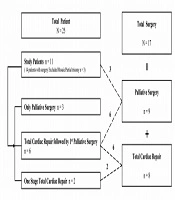
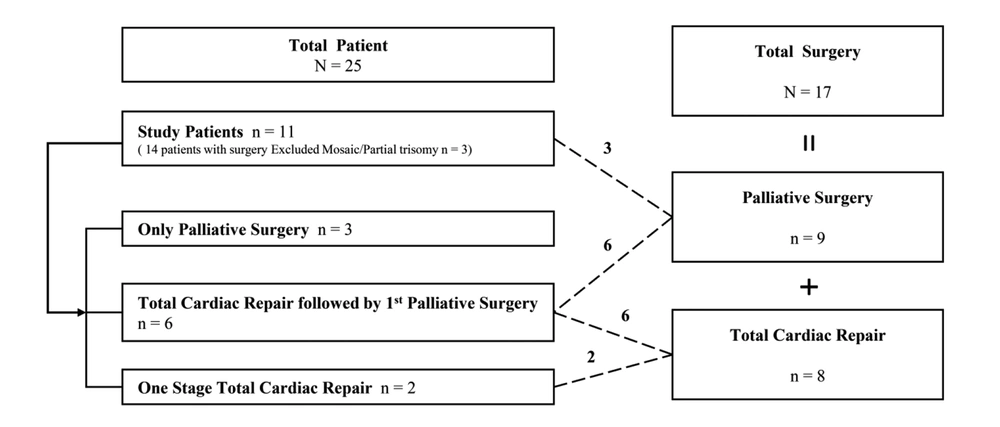
We reviewed the medical records of patients with T18 who had undergone cardiovascular surgery due to CHD at Samsung Medical Center between October 1995 and December 2019. Among 25 patients with T18, 14 underwent a cardiac operation. Three patients with low-level true mosaicism and partial trisomy were excluded because they can be associated with favorable fetal outcomes and improved survival (20). Finally, 11 with T18 underwent 17 cardiovascular surgeries: total cardiac repair with bypass in eight patients and palliative surgery without cardiopulmonary bypass in nine patients. Three of the 11 patients underwent surgery for palliation and eight patients for total cardiac repair. Total cardiac repair was followed by palliative surgery in six patients, and one-stage total cardiac repair was performed in only two patients (Figure 1). The decision for staged total cardiac repair was made after a discussion between pediatric cardiologists and surgeons. Usually, the reasons for palliative surgery were not only small body weight, small gestational age, and complex cardiac disease, but also all family member preference. Survival data were collected from all patients, and the preoperative, operative, and postoperative outcomes were obtained.
The collected data included demographics, initial cardiac diagnosis, extra cardiac anomalies, extra cardiac interventions, surgical procedure type, intraoperative details, postoperative complications, and length of hospital stay and mortality. Postoperative hospital mortality was defined as death before discharge from the hospital after surgery, and operative mortality was defined as a death within 30 days after cardiac surgery. The follow-up period was defined as the days from initial presentation until the last presentation documented in the medical record.
3.1. Statistical Analysis
Statistical analysis was performed using IBM® SPSS Statistics (version 24.0, IBM®, Armonk, NY). Comparisons were made between groups using Fisher’s exact test or Pearson’s chi-square test for dichotomous variables. Non-parametric testing with the Mann-Whitney U-test and Kruskal-Wallis test were utilized for continuous variables. Cumulative survival curves were made by Kaplan-Meier analysis. Statistical significance was established as P < 0.05.
This study was approved by the Institutional Review Board at Samsung Medical Center. The need for informed consent was waived by the board.
4. Results
Four (36.4%) of 11 patients survived, two after one-stage total cardiac repair (100%) and two after total cardiac repair followed by palliative surgery (33.3%). Zero patients undergoing only palliation survived. The median age of survivors was 827.5 (range, 413 - 1,647) days.
Patients undergoing palliative surgery, total cardiac repair followed by palliative surgery, and one-stage total cardiac repair along with their demographics, preoperative characteristics, and extra cardiac interventions are listed in Table 1. There were more female patients in all groups. The most common cardiac defect was VSD. Patients in the total cardiac repair group underwent tracheostomy and gastrostomy more often than patients in the other groups, followed by patients in the first palliative surgery group.
| Palliative Surgery (N = 3) | Total Cardiac Repair Followed by Palliative Surgery (N = 6) | One-stage Total Cardiac Repair (N = 2) | |
|---|---|---|---|
| Demographic factors | |||
| Male sex, n | 0 | 0 | 1 |
| IUGR, n | 3 | 4 | 1 |
| Birth weight, kg | 2.0 | 1.9 | 2.4 |
| Gestational age, wk | 39.4 | 37.8 | 39.2 |
| Maternal age, y | 33.0 | 32.0 | 33.0 |
| Follow-up, d | 90 | 360 | 805 |
| Age at 1st surgery, d | 7.0 | 11.0 | 43.5 |
| Weight at 1st surgery, kg | 1.5 | 2.2 | 3.0 |
| Cardiac diagnosis, n | |||
| VSD | 1 | 6 | 1 |
| PA with VSD | 0 | 0 | 1 |
| CoA/IAA with VSD | 1 | 0 | 0 |
| HLHS with IAA and VSD | 1 | 0 | 0 |
| Extracardiac anomalies, n | |||
| Renal | 0 | 1 | 0 |
| Neurologic | 1 | 0 | 0 |
| TE fistula | 0 | 2 | 0 |
| Gastrointestinal | 0 | 0 | 0 |
| Cleft lip/palate | 0 | 0 | 0 |
| Extracardiac interventions, n | |||
| Tracheostomy | 0 | 3 | 0 |
| Gastrostomy | 0 | 6 | 0 |
Abbreviations: CoA, coarctation of aorta; n, patient number; HLHS, hypoplastic left heart syndrome; IAA, interrupted aortic arch; PA, pulmonary atresia; SD, standard deviation; TE, tracheoesophageal; VSD, ventricular septal defect.
aContinuous variables are presented as median value.
Table 2 describes the cardiac diseases and surgery types in detail. Six patients initially underwent palliative pulmonary artery banding and then underwent complete repair with VSD and ASD closure. All but one cardiac surgery was performed after 2011.
| Patients | Cardiac Diagnosis | Firstoperative Procedure | Secondoperative Procedure |
|---|---|---|---|
| 1 | CoA VSD PDA | PDA ligation and PA banding and coarctoplasty | |
| 2 | HLHS IAA VSD PDA | PDA division and PA banding and Arch reconstruction &Atrial septectomy | |
| 3 | VSD ASD PDA | PDA ligation and PA banding | |
| 4 | VSD ASD PDA | PDA ligation and PA banding | VSD closure and ASD closure |
| 5 | VSD ASD PDA | PDA ligation and PA banding | VSD closure and ASD closure |
| 6 | VSD ASD PDA | PDA ligation and PA banding | VSD closure and ASD closure |
| 7 | VSD PFO PDA | PDA ligation and PA banding | VSD closure and PFO closure |
| 8a | VSD PDA | PDA ligation and PA banding | VSD closure and ASD closure |
| 9a | VSD PFO | PA banding | VSD closure and PFO closure |
| 10a | VSD ASD PDA | VSD closure and ASD closure and PDA ligation | |
| 11a | PA with VSD ASD PDA | VSD closure and ASD partial closure and PDA division and PA reconstruction |
Abbreviations: ASD, atrial septal defect; CoA, coarctation of aorta; HLHS, hypoplastic left heart syndrome; IAA, interrupted aortic arch; PA, pulmonary atresia; PDA, patent ductus arteriosus; PFO, persistent foramen ovale; VSD, ventricular septal defect.
aPatients were alive at last follow-up (for 413, 1647, 857, and 798 days, respectively).
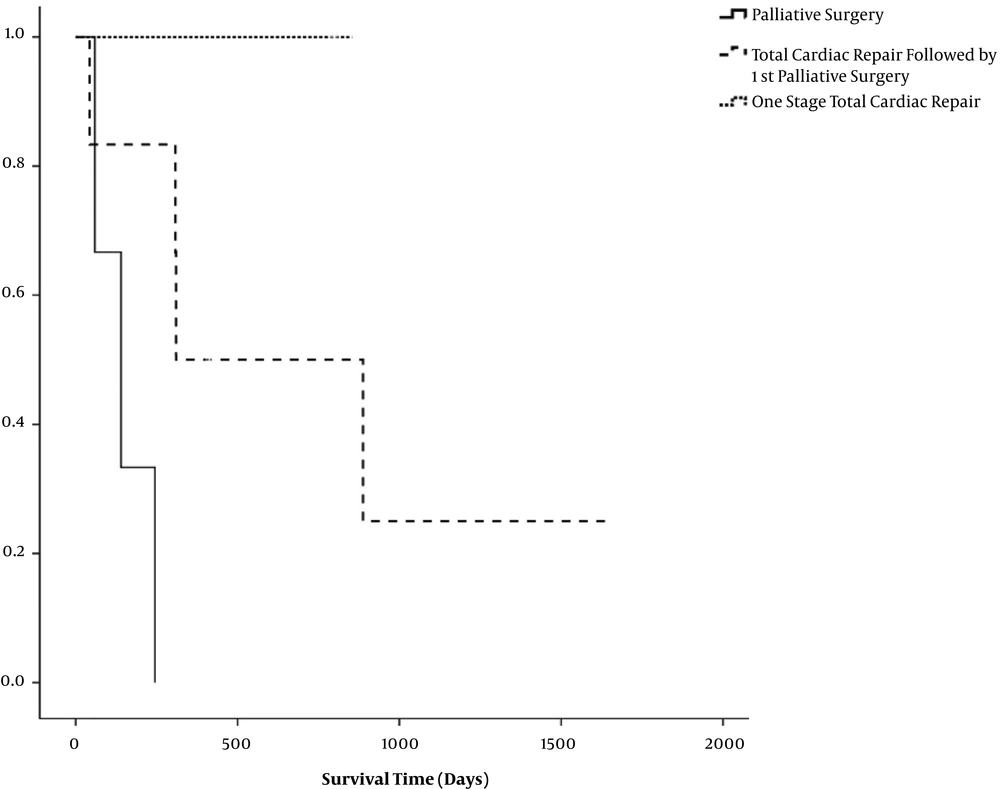
Figure 2 shows the cumulative survival for palliative surgery, total cardiac repair followed by palliative surgery, and one-stage total cardiac repair. At the last follow-up, both of two patients in the one-stage total cardiac repair were alive, whereas no patient in the only palliative surgery group was alive. The patients with total cardiac repair had better cumulative survival than those with palliative surgery. Survival curves in the palliative surgery groups showed a steep decline in survival in the first few months of life. Patients who underwent total cardiac repair were discharged after surgery and survived the next several months of life. The one-stage total cardiac repair patients were more likely to show a benefit for survival.
In Table 3, the outcomes and complications after each type of surgery (total cardiac surgery in eight patients vs palliative surgery in nine patients) are shown. Even though the ages and body weights at operation were different, the operative mortality, post-operative intensive care unit stay, and hospital stay were not statistically different. There was no difference in post-operative complications, such as cardiac, respiratory, renal, infectious, and neurologic problems.
| Palliative Surgery (N = 9) | Totalcardiac Repair (N = 8) | P-Value | |
|---|---|---|---|
| Age at operation, da | 8 (3 - 176) | 236 (15 - 601) | < 0.01 |
| Body weight at operation, ka | 2.1 (1.5 - 4.9) | 4.2 (1.9 - 10.5) | 0.03 |
| Outcomes | |||
| Postoperative ICU stay, da | 15 (2 - 80) | 10 (4 - 116) | 0.88 |
| Postoperative hospital stay, da | 70 (34 - 308) | 79 (11 - 373) | 0.81 |
| Postoperative mechanical ventilator support, da | 9 (3 - 70) | 12 (3 - 125) | 0.49 |
| Postoperative hospital mortality, No. (%) | 1 (11) | 2 (25) | 0.57 |
| Operative mortality, n | 0 | 0 | |
| Postoperative complication, No. (%) | 5 (55) | 4 (50) | 1.00 |
| Cardiac | |||
| Arrhythmia without pacemaker | 1 | 0 | |
| Pulmonary hypertensive crisis | 1 | 0 | |
| Cardiac arrest | 1 | 1 | |
| Respiratory | |||
| Chylothorax | 0 | 1 | |
| Pneumothorax | 1 | 1 | |
| Pleural effusion requiring drainage | 3 | 1 | |
| Pneumonia | 0 | 2 | |
| Respiratory insufficiency requiring re-intubation | 1 | 2 | |
| Phrenic nerve injury/paralyzed diaphragm | 3 | 1 | |
| Renal | |||
| ARF requiring temporary dialysis | 1 | 0 | |
| Neurologic | |||
| New onset seizures | 1 | 1 | |
| Infection | |||
| Sepsis | 0 | 2 |
Abbreviations: ARF, acute renal failure; ICU, Intensive Care Unit; n, patient number.
aValues are expressed as median (minimum-maximum).
Post-operative complications included pulmonary hypertension crisis, chylothorax, pneumonia, pleural effusion requiring drainage, pneumothorax, phrenic nerve injury, new onset seizure, paroxysmal supraventricular tachycardia, and acute renal failure requiring temporary dialysis. In addition, most surviving patients underwent gastrostomy due to feeding problems. The surviving patients were under home ventilation management after tracheostomy due to persistent, unexplained apnea symptoms.
5. Discussion
We observed that patients with T18 who underwent total cardiac repair for CHD had better survival than those undergoing palliative surgery. Regarding postoperative course and morbidity, palliative surgery was not superior to total cardiac repair with bypass.
Historically, surgery has not been frequently suggested due to the poor long-term prognosis in patients with T18. After the paradigm shift in treatment plan in 2005, many cardiac centers performed surgery (21), and many reported improved outcomes after cardiac surgery in T18 (11, 14-17). Our study also showed increased survival but relatively high mortality. In Japan, 1-year survival rates have been reported to be as high as 75% (18, 22, 23). A recent study found an overall 5-year survival rate of 12.3% for patients with T18 (16).
Furthermore, another study reported that all patients who underwent complete repair survived to discharge compared to only 57% of patients who underwent palliative surgery (P = 0.04) (17). Our study showed a similar result: 50% of the patients who underwent total cardiac repair survived to discharge compared to 0% of the only palliative surgery group (P = 0.03). However, patients in palliative surgery and total cardiac repair with or without palliation had a difficult postoperative course and complications. Moreover, they underwent other surgeries for combined non-cardiac anomalies.
Therefore, whether to perform cardiac surgeries for patients with T18 remains unclear, and little is known about factors that affect outcomes. For better survival in the present study, patients must have undergone tracheostomy and gastrostomy in addition to cardiac surgery as well as multiple visits to outpatient clinics and the Emergency Department. Patients with T18 have improved long-term outcomes with intensive treatment, including surgical correction of gastrointestinal malformations, respiratory support, and pharmacological treatment for congenital heart defects (11, 12, 14, 15). Therefore, the results from this study demonstrated that T18 patients can undergo successful cardiac surgeries with improved survival rates following careful case selection, assessment of procedural benefits and risks, and consideration of other combined anomalies (except cardiac problems) and counseling by a team of experts. Long-term follow-up of patients who undergo cardiac surgery is necessary to determine survival and improve quality-of-life issues for these patients and their families.
The present study showed relatively high mortality in the long term. Nevertheless, patients with T18 and their families should be given the option for surgery to improve their quality of life. This study is valuable to clinicians and families to assist in counseling and deciding which interventions to offer. Physicians must address the issue of improved quality of life after cardiac surgery and inform families about diminished survival and suspected poor quality of life from recommending complex procedures in addition to cardiac surgery. In particular, the concept of ‘quality of life’ is controversial because of possible poor hospital courses and other complications. Treatment of congenital heart disease associated with syndromes with genetic defects is a debated issue (24). Traditionally, non-intervention in neonate management of T18 has been common. Bos et al. (25) reported ethical issues, stating that early diagnosis is important to avoid unnecessary and emergency surgery in newborns with trisomy 18.
However, the recent data show an improvement of the overall surgical results (11, 12, 14-17). The complexity of cardiac disease must be considered when determining whether to operate. In the present study, most patients who underwent surgery had simple VSD, and the percentages of patients with complex heart disease, such as pulmonary atresia (PA) with VSD, coarctation of the aorta with VSD, and hypoplastic left heart syndrome with interrupted aortic arch and VSD were small (27%). There were 3 patients with simple VSD and 1 patient with PA with VSD alive at the last follow up. The patients with complex heart disease showed quite an unfavorable short-to-mid-term prognosis and were usually considered unsuitable for repair of associated cardiac malformations.
Cereda and Carey (26) revealed that the major causes of death were central apnea, cardiac failure due to cardiac malformations, sepsis with aspiration, upper airway obstruction, respiratory insufficiency due to hypoventilation, and, likely, the combination of these and other factors. It is likely that upper airway obstruction is more common than previously realized. In the present study, 10 of 25 patients died of respiratory failure, not cardiac failure. Therefore, patients and their families should be offered a multidisciplinary care team to provide information to make the best decision possible for T18 patients. Patients must be evaluated on a case-by-case basis to determine the best clinical management plan. The complexity and severity of clinical presentation at birth must be considered. Patients with T18 should receive multiple pediatric and specialist evaluations, especially in the first 12 months of life.
In conclusion, the present study demonstrated that patients with T18 who underwent total cardiac repair for CHD and one-stage total cardiac repair were more likely to benefit in terms of survival. Even patients undergoing palliative cardiac surgery had a difficult postoperative course and suffered complications similar to those with bypass total correction. Therefore, every surgical intervention should be considered after comprehensive communication among medical persons and parents.