1. Background
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) emerged in China in December 2019 and spread worldwide, leading to a significant public health problem (1). The virus is highly contagious and can spread through exposure to respiratory droplets and close contact among human beings. It can affect people of all age groups. Pregnant women and their neonates are at risk of COVID-19 because of their unique physiologic and immunologic status. Despite the increasing number of COVID-19 patients, there is only a little information available concerning its effects on pregnant women and their infants.
2. Objectives
This paper retrospectively evaluated clinical data, laboratory findings, and outcomes of neonates born to affected mothers with SARS-CoV-2 in five hospitals in Tehran, Iran, from March 20 to September 5, 2020.
3. Methods
To meet the requirements of research, a cohort study was conducted on all neonates born to mothers with positive real-time reverse transcriptase-polymerase chain reaction (RT-PCR) SARS-CoV-2 or clinically suspected COVID-19. Neonates enrolled in this study were from five different hospitals affiliated with the Tehran University of Medical Sciences (Imam Khomeini, Shariati, Yas, Arash, and Ziaeian hospitals) in Tehran, Iran, between March 20 and September 5, 2020. The survey was approved by the Medical Ethics Committee of Tehran University of Medical Sciences (code: IR.TUMS.VCR.REC.1399.017), and informed consent forms were signed by the parents or caregivers of newborns. The medical records of all the neonates born to mothers with positive RT-PCR or clinically suspected with chest computed tomography (CT) were examined, and they were tested for COVID-19 infection and followed up for their outcomes. The nasopharyngeal swabs were done for the RT-PCR assay for SARS-CoV-2 in all neonates born to the confirmed or suspected mothers for the first time 24-48 hours after birth and the second time as indicated at subsequent visits. The neonatal sampling was standardized for sufficient specimens (2).
3.1. Data Collection and Analysis
A questionnaire was designed by the researcher for obtaining obstetric and delivery history and neonatal data, including gestational age, birth weight, sex, mode of delivery, Apgar score, clinical features, RT-PCR test results (for both mothers and neonates), laboratory tests, radiologic findings, hospital admissions, length of stay, management, and outcomes. All the related data were extracted from the electronic service systems in the hospitals.
3.2. Statistical Analysis
The study aimed to evaluate the clinical features and laboratory data of neonates born to mothers with COVID-19 (positive test or suspected with chest CT), looking for a relationship between the positive RT-PCR test result of the neonates and the type of clinical features and the percentage of neonates with COVID-19 infection who were born to infected mothers. To conduct a comprehensive analysis, all categorical data were manifested as frequency (%) and continuous data as mean ± SD. The statistical analyses were fulfilled with the IBM SPSS ver. 18.0 Statistical Package for Windows.
4. Results
Parallel to the research objectives, the following results emerged from 44 neonates (35 singletons, three twins, and one triple) born to 39 infected mothers during the study period. Thirty-five pregnant women had confirmed COVID-19 with a positive RT-PCR test, and four mothers were clinically suspected by their chest CT, which had a suggestive pattern of SARS-CoV-2. Nineteen women had complications during pregnancy, including hypertensive disorder, gestational diabetes, premature rupture of membrane, and hypothyroidism (Table 1). Out of the 44 neonates, 26 (59%) were males, 33 (75%) were delivered by cesarean sections, 20 (45.5%) were term, and 24 (54.5%) were preterm. The mean gestational age and birth weight were 35.11 ± 4.01 weeks (range: 26 - 40) and 2,567 ± 898 g (range: 900 - 4200), respectively. Most of the infants had a good Apgar score at one and five minutes. All the newborns were tested for SARS-CoV-2 using nasopharyngeal swabs during the first 24-48 hours of life, which was positive in two neonates. Also, seven newborns were positive in the second time swabbing, ranging from 2 to 16 days after birth. One case tested positive both at the first and second time of swabbing, so a total of eight (18.1%) infants had positive tests.
| Maternal Characteristics | Values (N = 39) |
|---|---|
| Nasopharyngeal PCR positive for SARS-CoV-2 | 35 (89.7) |
| Clinically suspected, confirmed by CT for SARS-CoV2 | 4 (10.3) |
| Duration of symptoms before delivery in mothers, d | 5.63 (2.37) |
| Past medical history | |
| High blood pressure | 2 (5.1) |
| Gestational diabetes mellitus (GDM) | 4 (10.2) |
| Premature rupture of the membranes (PROM) | 6 (15.4) |
| Hypothyroidism | 7 (18) |
| None | 20 (51.3) |
| Intensive care unit admission | 6 (15.38) |
| Death | 3 (7.69) |
aValues are expressed as No. (%) or mean ± SD.
Droplets and contact precaution guidelines were tightly controlled and followed in all the hospitals for the delivery room, equipment, and transferring newborns to the well-baby nursery or NICU. Twenty-nine (65.9%) neonates were asymptomatic at birth, being kept with their mothers in an isolated situation maintaining a physical distance of 180 cm, except for those six neonates whose mothers needed Intensive Care Unit (ICU). Fifteen (34.1%) neonates were symptomatic at birth and admitted to the NICU. During the observation, more neonates became symptomatic. Finally, 27/44 (61.3%) neonates became symptomatic, and 17/44 remained asymptomatic.
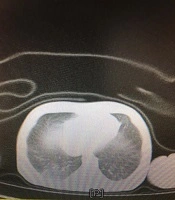
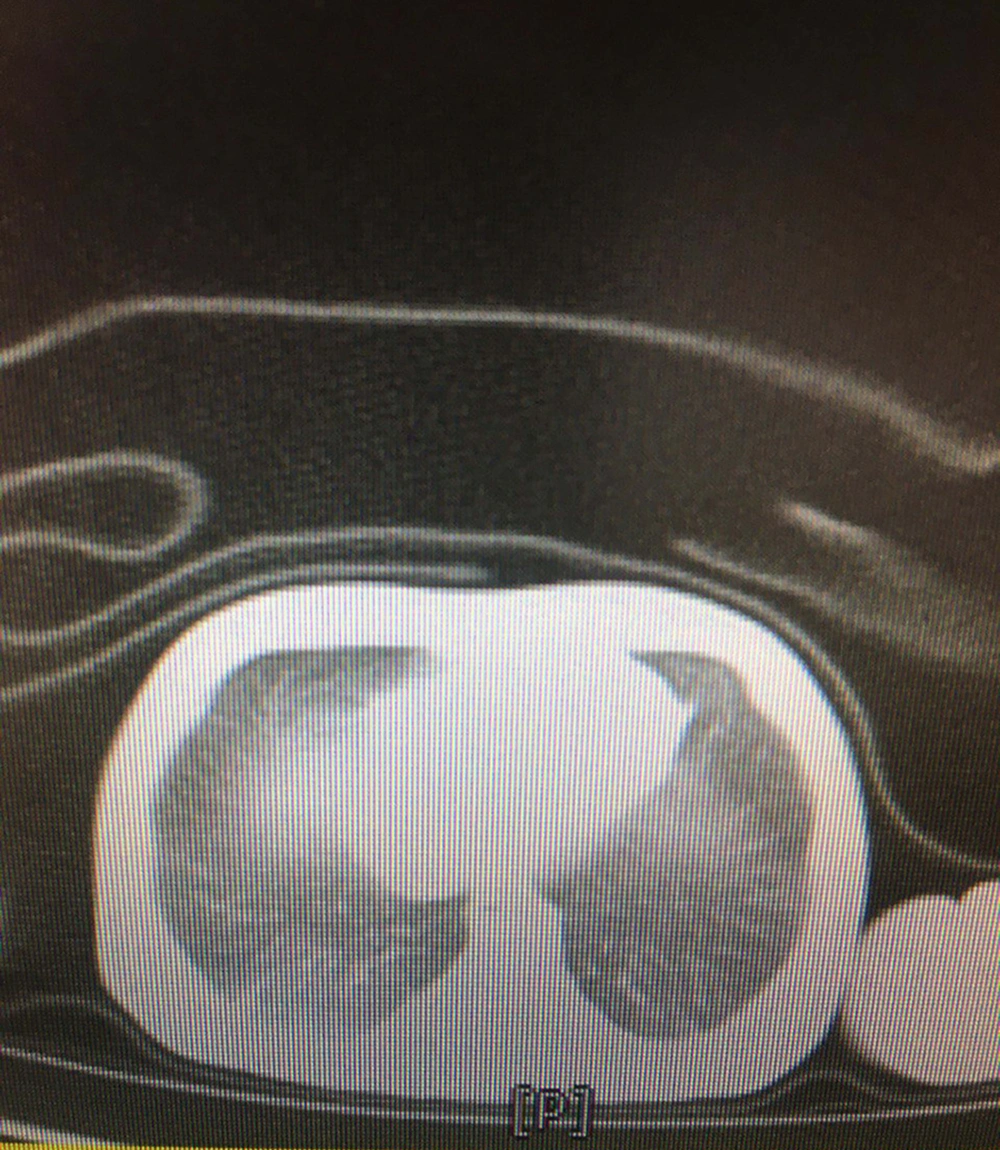
The most common clinical manifestations were respiratory distress (77.7%), followed by fever or hypothermia (18.5%), gastrointestinal problems (14.8%), and neurologic findings (3.7%). Among them, four neonates were found with a combination of symptoms. The demographic and clinical characteristics of the neonates are presented in Table 2. Few abnormalities were seen in laboratory findings. The C-reactive protein was increased in 10 (22.7%) neonates, with 3/27 (11.1%) having coagulative disorders. Lactate dehydrogenase was elevated in 12 out of 16 (75%) neonates. Besides, aspartate aminotransferase was increased in five out of 12 (41.6%) newborns, and 16/22 (72.7%) neonates had pathologic hyperbilirubinemia that was managed according to their hour-specific age. None of the neonates had leukocytosis or leukopenia, lymphopenia, thrombocytosis, or thrombocytopenia. All the cultures were negative. The chest X-rays were abnormal in 6/27 (22.2%) neonates, including ground-glass opacities (18.5%) and bilateral consolidations (3.7%). A lung CT scan was done for one of the neonates with respiratory symptoms, who had three negative RT-PCR tests but was highly suspicious of COVID-19 pneumonia. The pattern was suggestive of COVID-19 pneumonia reported by the consultant radiologist (Figure 1).
| Variable | Patients/Value (N = 44) |
|---|---|
| Newborn features | |
| Gestational age, wk | 35.11 ± 4.01 |
| Birth weight, g | 2567 ± 898 |
| Apgar score in minute 1 | 7.18 ± 2.03 |
| Apgar score in minute 5 | 8.50 ± 1.64 |
| Sex | |
| Female | 18 (41) |
| Male | 26 (59) |
| Delivery | |
| C/S | 33 (75) |
| Normal vaginal delivery | 11 (25) |
| Nasopharyngeal PCR positive for SARS-CoV-2, first time | 2 (4.5) |
| Nasopharyngeal PCR positive for SARS-CoV-2, second time | 7 (15.9) |
| Nutrition | |
| Breastfeeding | 5 (11.4) |
| Formula | 39 (88.6) |
| Asymptomatic at birth | 29 (65.9) |
| Remained asymptomatic during observation | 17 (38.6) |
| Symptomatic | 27 (61.4) |
| Symptoms | |
| Respiratory distress, % | 77.7 (21/27) |
| Gastrointestinal problems, % | 14.8 (4/27) |
| Fever or hypothermia, % | 18.5 (5/27) |
| Neurologic findings, % | 3.7 (1/27) |
aValues are expressed as No. (%) or mean ± SD.
The management and outcome of neonates are demonstrated in Table 3. Out of 29 neonates who were asymptomatic at birth, 12 became symptomatic during observation and were admitted, whereas 17 remained asymptomatic and received supportive care. Among symptomatic newborns, 44.4% (12/27) were intubated and received mechanical ventilation. Seven newborns needed non-invasive ventilation as non-invasive positive-pressure ventilation (NIPPV) or continuous positive airway pressure (CPAP), and two patients required oxygen therapy with an oxy hood.
| Values | |
|---|---|
| Newborn age on admission to NICU, d | 1.36 ± 1.47 |
| Duration of hospitalization, d | 4.62 ± 5.98 |
| Supportive treatment | 17 (38.6) |
| Mechanical ventilation | 12/27 (44.4) |
| NIPPV or CPAP | 7/27 (25.9) |
| O2 therapy with oxyhood | 2/27 (7.4) |
| Duration of mechanical ventilation, d | 3.41 ± 8.39 |
| Antibiotics | 27 (61.4) |
| Surfactant | 3/27 (11.11) |
| Death | 1 (2.3) |
aValues are expressed as No. (%) or mean ± SD.
Empiric antibiotics were prescribed for 27/44 (61.4%) of symptomatic neonates, and surfactant replacement therapy was used in three cases. According to the Pediatric Infectious Disease Department’s consultation, five (18.5%) newborns with massive secretions, high oxygen requirement, and ventilator dependency with an unfavorable response to antibiotics were treated with hydroxychloroquine. Their general conditions improved. Hydroxychloroquine was advocated for the treatment of SARS-CoV-2 at first in the pandemic; these five neonates were treated with hydroxychloroquine in that period. In our study, there were three maternal deaths and one neonatal death.
5. Discussion
According to the spread of SARS-CoV-2 worldwide, there is a significant concern about its effect on pregnant women and their offspring. To date, despite the belief in lighter clinical features, fewer laboratory and radiologic findings, and better outcomes in children than in adults (3-6), some data have explained that infants with COVID-19 may have more severe symptoms than children (7). As the information on neonatal SARS-CoV-2 is limited, we herein presented a multicenter study on newborn infants born to mothers with confirmed or suspected COVID-19 in Iran.
In our study, 54.5% of the neonates were preterm with a mean gestational age of 35.11 weeks, which was similar to the mean gestational age of 34 weeks reported by Patil et al. (8). Schwartz et al. found that 63% of neonates with COVID-19 were delivered before 37 weeks of gestation (3). Zhu et al. (9) reported that of 10 neonates born to nine mothers with COVID-19, six were preterm. Other studies reported preterm deliveries in 21.2% to 35% of pregnant women with the symptoms of COVID-19 (5, 10, 11). Although it is hypothesized that SARS-CoV-2 may cause hypoxia during pregnancy, which may result in an increased risk of preterm delivery (5), it should be considered that COVID-19 may exacerbate pregnancy complications, including preeclampsia, coagulative disorders, thromboembolic events, and cardiomyopathy, which may lead to preterm births (3). Moreover, further data are needed to claim that maternal COVID-19 can lead to preterm birth.
Neonatal morbidities, including low birth weight and small for gestational age, may be induced by viral infections. Moreover, in the present study, the mean birth weight of the neonates was 2,567 g. A systematic review by Yoon et al. (5), including 201 neonates born to mothers with COVID-19, reported low birth weight and small for gestational age in 15.6% and 8.3% of the cases, respectively. Dubey et al. (12) analyzed 548 neonates from 61 studies and estimated the rates of premature birth and low birth weight as 23% and 7%, respectively. Another systematic review showed the rates of preterm neonates and small for gestational age as 23.8% and 11.2%, respectively (13).
Of note, in our study, males were more affected than females, which is supported by other studies (8, 14-16). The male propensity of the disease needs more investigation. Newborn infants may be affected by SARS-CoV-2 through vertical or horizontal transmission. We reported two infants (4.5%) with positive SARS-CoV-2 results taken by a nasopharyngeal swab within the first 24 hours of birth despite the use of preventive measures during and after delivery and seven cases (15.9%) who had positive tests in the second sampling obtained between two and 16 days of life. In several case reports, the vertical transmission was suspected if a neonate was tested positive for COVID-19 RT-PCR within the first 48 h of birth in the presence of preventive measures during delivery (16). Besides, Zeng et al. (17) reported out of 33 neonates born to SARS-CoV-2-positive mothers, three had positive nasopharyngeal and anal swabs on days 2 and 4. Zamaniyan et al. (18) reported a preterm infant born to a mother with COVID-19, who had a positive nasopharyngeal swab at 48 h. His amniotic fluid was tested for SARS-CoV-2 by RT-PCR, which was also positive. However, false-positive results for RT-PCR on nasopharyngeal specimens were described (19, 20). Also, Chi et al. (13) described 91 neonates tested for SARS-CoV-2, and 8.8% of the neonates had a positive nucleic acid or antibody test result, indicating that the possible risk of vertical transmission should be considered. Sheth et al. (16) reported 10/326 (3%) neonates, with the possibility of acquiring SARS-CoV-2 through vertical transmission. All the 120 neonates born to mothers with positive SARS-CoV-2 in the Salvatore et al. (21) survey were asymptomatic and did not have a positive test on the first day, nor at 5-7 days or 14 days after birth. No vertical transmission of SARS-CoV-2 from the mother to the neonate was demonstrated by Dumitriu et al. (22). As vertical transmission could not be totally confirmed or ruled out, further research regarding this issue is warranted. Neonates born to SARS-CoV-2-infected mothers may be asymptomatic or may present with mild symptoms. Gotzinger et al. (7) described that infants younger than one month may have more severe manifestations than older children. In the present study, about 40% of the neonates had no symptoms at birth and remained asymptomatic during the follow-up period. This result is much lower than that reported by Salvatore et al. (21) and other studies, in which all the neonates born to mothers with positive SARS-CoV-2 RT-PCR tests were asymptomatic (21, 23-25). Additionally, a review by Sheth et al. (16) reported that 30% (7/23) of COVID-19-positive neonates were asymptomatic; also, in a study by De Bernardo et al., 4/25(16%) were asymptomatic (15).
Our study revealed that 15 (34.1%) neonates were symptomatic at birth and were admitted to the NICU. This number increased during the observation period and reached 27 (61.4%) newborn infants who became symptomatic later on and were admitted. The most common symptom was respiratory distress (77.7%), temperature instability including fever or hypothermia (18.5%), gastrointestinal disorders (14.8%), neurologic findings (3.7%), and others. Like our study, respiratory distress was the most frequent manifestation (63%) in Schwartz et al.’s research (3). However, Zimmermann and Curtis (6) illustrated different neonatal features in 67 babies born to mothers with confirmed COVID-19 infection, including respiratory distress and pneumonia in 18%, disseminated intravascular coagulation in 3%, asphyxia in 2%, as well as two perinatal deaths. The most common clinical features of neonates with positive SARS-CoV-2 RT-PCR in the Yoon et al.’s study (5) were vomiting (75%) and the signs of pneumonia as fever and shortness of breath (50%). In the De Bernardo et al.’s study (15), fever (28%) was the most common manifestation at the onset. The neonatal symptoms may vary according to different races and geographic regions. In the current study, of eight neonates with positive RT-PCR, 87.5% had respiratory distress, 12.5% presented with neurologic symptoms, 12.5% temperature instability, 12.5% gastrointestinal disorder, and 12.5% were asymptomatic. Notably, the rate of neonates with positive COVID-19 RT-PCR assay without any symptoms was lower in our study than in De Bernardo et al. (16%) (15) and Sheth et al.’s (16) (30%) studies.
Minor laboratory abnormalities were observed in our research, including elevated CRP, increased lactate dehydrogenase (LDH), mild coagulopathy, and abnormal liver function tests. We did not find any abnormalities in the complete blood count (CBC) test. In comparison, some studies showed leukocytosis/leukopenia, lymphopenia, and thrombocytosis (26). Nonspecific changes have been mentioned in other studies (16, 17).
Moreover, the majority of the lung images were normal in our study, but the most common abnormalities were ground-glass opacities in 18.5% and bilateral consolidation in 3.7% of the newborn infants, respectively. Positive findings in chest X-rays have been described in 56% of neonates as the ground-glass appearance in 28% of cases (26). In a systematic review among 68 neonates, 26.5% of babies showed radiologic signs of pneumonia (5). Other radiologic findings, including increased lung marking, thickened texture, or high-density nodular shadow, have been reported in SARS-CoV-2-positive newborns. It seems that radiological features in neonates are similar to those of older children and adults (27).
In general, 27 (61%) symptomatic neonates were admitted, and 34.1% received care in the NICU for their disease severity. Our results were compatible with Gale et al. (26) study that reported 64% of the 66 neonates with SARS-CoV-2 infection were admitted, 30% received care in a neonatal unit, and 6% in a Pediatric Intensive Care Unit (PICU). Severe disease was observed in 42% of the neonates, of whom 36% received critical care or respiratory support (26). In our study, 47.2% (21/44) of the patients required respiratory support. The rate of respiratory support was higher in our study than in the Gale et al. (26) study in which 33% of the neonates received respiratory support, including 4.5% invasive, 15.1% noninvasive, and 33% supplemental oxygen. In addition, one-third of the neonates in a systematic review by Juan et al. (28) were admitted to the Intensive Care Unit. The NICU admission rate of 38.3% was reported in other studies (29). Viral respiratory infections in neonates may increase the need for critical care and respiratory support. Additionally, viral infections may induce preterm birth, and prematurity itself may require respiratory support.
In the current study, all the symptomatic neonates received antibiotics. Hydroxychloroquine was administered to five patients. A few studies reported treatment with oral hydroxychloroquine and/or azithromycin and other antiviral agents (26, 29). There is no definite recommendation for neonatal treatment. To date, supportive care, including fluid, caloric intake, and oxygen supplements, is the mainstay of management.
All the mothers had symptoms within seven days of delivery, with a mean duration of 5.63 days. They had a higher rate of perinatal complications in comparison with Zimmermann and Curtis (6) study subjects, including ROM (15.4 % vs. 12%), GDM (10.2% vs. 5%), and hypothyroidism (18% vs. 3%), except for the lower rate of gestational hypertension (5.1% vs. 6%). Hypertensive disorders (5.1%) were similar to Yan et al.’s study (11) rate (4.3%), but the GDM rate in our study was higher (10.2% vs. 7.8%). Trocado et al. (10) reported lower adverse pregnancy complications consisting of PROM (5%), GDM (3%), and gestational hypertension (2%). It is supposed that SARS-CoV-2 infection may exacerbate adverse pregnancy outcomes in pregnant women.
The ICU admission of mothers with COVID-19 was 15.38% in our study, which was higher than in Yoon et al. (5), Patil et al. (8), and Yan et al. (11) research that showed the rates of 4.9%, 4.4%, and 6.9%, respectively. The mortality rate of mothers with COVID-19 was 7.69%, which was much lower than the 25% mortality rate of mothers with SARS demonstrated by Wong et al. (30). In contrast to our results, none of the mothers with SARS-CoV-2 died in Yan et al.’s study (11).
We had one neonatal death (2.2%) in our study. This neonate had her first SARS-CoV-2-positive test on the 16th day of life and a negative test on day 24. Her mother had two previous deaths in her children. They died with a suspicious diagnosis of spinal muscular atrophy (SMA). This baby also had spasticity and contracture anomalies in her limbs with high serum ammonia levels. She was intubated for 41 days because of poor respiratory effort. Unfortunately, she died in 44 days of her life because of severe respiratory failure with no definite diagnosis. We assume that her death was due to her underlying diseases and prematurity rather than COVID-19.
All the neonates were followed up. The eight neonates with SARS-CoV-2-positive tests had negative results approximately within 10 days after a positive SARS-CoV-2 test, similar to De Bernardo et al.’s survey (15), in which swabs became negative in 10.3 ± 4.5 days. All were discharged from the hospital in good condition, except one who died, as explained before.
Our research limitation was the small sample size, a short follow-up period, and repeated updating of guidelines for neonatal management due to the novelty of SARS-CoV-2. However, we recruited all the neonates born to mothers infected with COVID-19 during 5.5 months in five different hospitals in Tehran, Iran. To our knowledge to date, this survey is the most considerable multicenter research about neonatal COVID-19 infection in Iran. The pandemic’s dynamic could lead to changes in the current data, so updating the knowledge with larger sample size and a more extended follow-up period in this regard is encouraged.
5.1. Conclusions
The SARS-CoV-2 infection during pregnancy may cause severe maternal and neonatal morbidities, including preterm delivery. However, we had no control group to verify if preterm birth was due to mothers’ co-morbidities or COVID-19 infection. Neonates with positive SARS-CoV-2 test may demonstrate a spectrum of clinical features, laboratory, and radiographic findings. The awareness of health care providers and updating their knowledge may help with the accurate diagnosis and appropriate management of this infection as soon as possible.
Precise monitoring and future surveillance studies are needed to evaluate the long-term outcomes of infected neonates.