1. Background
Systemic lupus erythematosus (SLE) is an autoimmune disorder characterized by auto-antibodies against self-antigens (1). Hormonal, environmental and genetic factors may contribute to the development of SLE (2).
It is evident that disturbances in the immune regulation mechanisms, such as imbalance of pro-inflammatory and anti-inflammatory cytokines, contribute to the development of SLE (1).
Interleukins (ILs) are a large group of immunomodulatory proteins that elicit a wide variety of responses in cells and tissues (3). Interleukin 37 (IL-37) was first described in 2000 as IL-1 family member 7 (IL-1F7). It maps to the IL-1 family cluster of genes on chromosome 2 (4).
IL-37 is a key cytokine in regulating inflammation. Its expression in macrophages or epithelial cells inhibits the synthesis of pro-inflammatory cytokines. In addition, IL-37 protein can be up-regulated by pro-inflammatory cytokines and inflammatory stimuli (5).
It is demonstrated that the expressions of pro-inflammatory cytokines TNF-α, IL-6 and IL-1β were suppressed by IL-37 in active SLE patients, thereby IL-37 is an important cytokine in the control of SLE pathogenesis by suppressing the production of inflammatory cytokines (6).
This study aimed to detect the level of IL-37 in SLE patients and to correlate it with disease activity.
2. Methods
This cross-sectional study included fifty children of both sexes, with SLE following up at the pediatric rheumatology clinic, children’s hospital, Cairo University, aged up to 16 years. Thirty apparently healthy age- and gender-matched children with no family history or clinical manifestation suggestive of SLE or any rheumatological disorders, served as controls.
SLE cases were diagnosed according to Systemic Lupus International Collaborating Clinics (SLICC) group classification criteria for SLE (7).
Patients diagnosed with other vascular or collagen diseases (as familial Mediterranean fever or juvenile idiopathic arthritis) and patients diagnosed with other conditions associated with high level of IL-37 as Guillain-Barré syndrome or cancers were excluded from the study.
2.1. The Following Data Were Collected from Patients and Their Files
1- Proper history taking including demographic data as age, sex, age at diagnosis, history of the disease at its onset and its presenting manifestation, the dose of steroids and other immunosuppresants at the time of study. Long-term use of steroids was considered when used more than 60 days. Disease activity at the time of the study was measured using SLEDAI-2K score of activity in all patients (8).
2- Full physical examination included weight, height, body temperature, blood pressure and presence of edema, vasculitis or arthritis.
3- Laboratory investigations: complete blood count (CBC), erythrocyte sedimentation rate (ESR), urine analysis, complement 3 (C3) and complement 4 (C4) were collected from the patients at the time of the study.
The Kit used for measurement of serum IL-37 level by enzyme-linked immunosorbent assay (ELISA) was provided by EIAab, E10078h, Wuhan EIAab Science Co., Ltd.
2.2. Statistical Analysis
Excel computer program was used to tabulate the results, and represent it graphically. Quantitative variables were expressed as mean and standard deviation. Qualitative variables were expressed as count and percent. One Way ANOVA was used to declare the significant difference between groups at P < 0.05. Duncan multiple comparison test at P < 0.05 was used to declare the significance between two groups. Chi square test was used to declare the significance difference in the distribution between groups at P < 0.05. Non-parametric test (Mann Whitney test) was used to compare between cases and controls regarding IL-37 level using median and interquartile range (IQR) (9).
3. Results
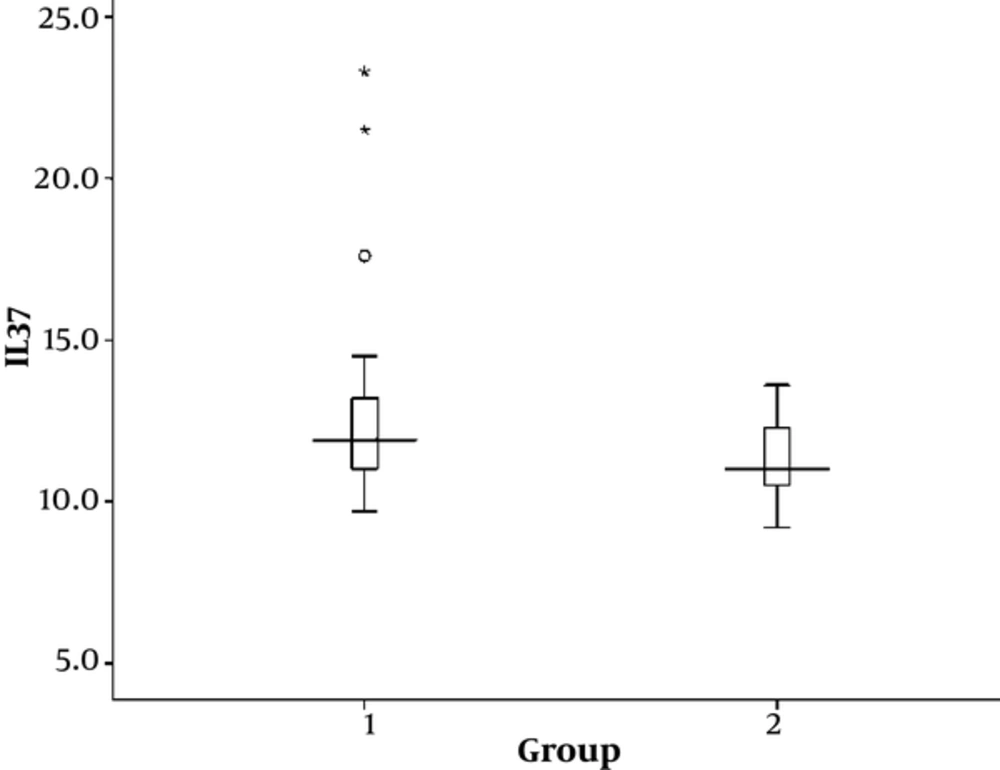
Out of 50 cases, 39 (78%) were females and 11 (22%) males. Out of 30 controls, 21 (70%) were females and 9 (30%) males. The level of IL-37 was higher in cases (18.66 ± 28.93) than in control group (11.40 ± 1.12). The IL-37 levels are also compared between cases and controls using non-parametric test. We found the median of IL-37 in cases was 11.9 with the interquartile range (IQR) 11-13.2, while the median of IL-37 in controls was 11 with the IQR 10.63-12.3, with P value of 0.028 which was statistically significant (Figure 1).
Demographic data and duration of disease of cases is summarized in Table 1. Clinical and laboratory data of cases at diagnosis and at the time of study are shown in Table 2 and Table 3, respectively.
| Variables | Minimum | Maximum | Mean ± SD |
|---|---|---|---|
| Age, y | 9 | 16 | 13.62 ± 1.96 |
| Age at onset of disease, y | 2 | 14 | 9.48 ± 2.44 |
| Age at diagnosis, y | 4 | 15 | 10.12 ± 2.44 |
| Duration between onset and diagnosis, days | 12 | 1460 | 267.14 ± 338.66 |
| Disease duration, mo | 2 | 168 | 49.38 ± 37.79 |
Demographic Data and Duration of Disease in Cases
| Variables | Frequency | Percentage, % |
|---|---|---|
| Family history | 7 | 14 |
| Fever | 39 | 78 |
| Arthritis | 35 | 70 |
| Skin manifestation | 27 | 54 |
| Neurological manifestation | 8 | 16 |
| Serositis | 8 | 16 |
| Edema | 7 | 14 |
| Positive ANA | 40 | 80 |
| Positive ant-DNA | 20 | 40 |
| Anemia | 17 | 34 |
| Thrombocytopenia | 8 | 16 |
| Leukopenia | 7 | 14 |
Clinical and Laboratory Data of Cases at Diagnosis
| Variables | Minimum | Maximum | Mean ± SD |
|---|---|---|---|
| Weight, kg | 21 | 83 | 47.07 ± 13.28 |
| Height, cm | 122 | 165 | 146.86 ± 9.75 |
| SLEDAI-2K score | 0 | 15 | 4.60 ± 3.22 |
| ESR | 4 | 125 | 33.52 ± 30.29 |
| Hemoglobin, g/dL | 8.9 | 14.2 | 11.84 ± 1.41 |
| TLC, thousands/cmm | 1.9 | 16.6 | 7.29 ± 3.51 |
| Platelet count, thousands/cmm | 128 | 608 | 299.72 ± 88.29 |
| Level of IL-37 | 9.7 | 183.4 | 18.66 ± 28.93 |
Clinical and Laboratory Data of Cases at the Time of Study
Out of 50 cases, 18 (36%) were presented without lupus nephritis, 5 (10%) presented with class I lupus nephritis, 12 (24%) presented with class II lupus nephritis, 7 (14%) presented with class III lupus nephritis and 8 (16%) presented with class IV lupus nephritis.
Out of 50 cases, 48 (96%) used steroids for long term period. Doses of steroids ranged between 1.3 to 55 mg/day with a mean of 10.98 ± 9.23 mg. Forty seven (94%) used hydroxychloroquine with doses ranging between 100 and 400 mg/day with a mean of 202.13 ± 32.90. Nine (18%) used mycophenolate mofetil with doses ranging between 1000 and 1500 mg/day with a mean of 1416.67 ± 176.78. Thirty one (62%) used cyclophosphamide and 19 (38%) used azathioprine.
We classify cases according to SLEDAI-2K score into two groups: active group which represents 34 (68%) cases and inactive group which represents 16 (32%) cases. Comparison between active and inactive cases according to demographic data, disease duration, clinical manifestations and laboratory investigations are shown in Tables 4 and 5. The level of IL-37 was higher in active group than in inactive group, however with no statistically significant value (P = 0.580). Consumed C3 and C4 were significantly more frequent in active group.
| Variables | Active (N = 34) | Inactive (N = 16) | P Value |
|---|---|---|---|
| Age, y | 13.32 ± 2.14 | 14.25 ± 1.34 | 0.171 |
| Age at onset of disease, y | 9.09 ± 2.66 | 10.31 ± 1.70 | 0.152 |
| Age at diagnosis, y | 9.74 ± 2.53 | 10.94 ± 1.61 | 0.117 |
| Duration between onset and diagnosis, day | 271.32 ± 358.93 | 258.25 ± 301.84 | 0.832 |
| Disease duration, mo | 50.38 ± 42.82 | 47.25 ± 24.97 | 0.706 |
| Hgb (g/dL) at the time of study | 11.62 ± 1.49 | 12.31 ± 1.14 | 0.131 |
| TLC (thousands/cmm) at the time of study | 7.17 ± 3.85 | 7.53 ± 2.76 | 0.405 |
| Platetelet count (thousands/cmm) at the time of study | 295.59 ± 96.14 | 308.50 ± 70.78 | 0.567 |
| ESR at the time of study | 37.65 ± 33.57 | 24.75 ± 19.95 | 0.278 |
| Level of IL-37 at the time of study | 21.41 ± 34.83 | 12.81 ± 3.35 | 0.580 |
| Dose of steroids, mg | 13.24 ± 10.22 | 6.17 ± 3.52 | 0.002a |
| Dose of Mycophenolate moftil, mg | 1458.33 ± 102.06 | 1333.33 ± 288.68 | 0.480 |
| Dose of Hydroxychloroquine, mg | 196.97 ± 17.41 | 214.29 ± 53.45 | 0.126 |
Comparison Between Active and Inactive Cases According to Demographic Data, Disease Duration, Laboratory Investigations and Treatment
| Variables | Active (N = 34) | Inactive (N = 16) | P Value |
|---|---|---|---|
| At diagnosis | |||
| Fever | 28 (82.4) | 11 (68.8) | 0.297 |
| Arthritis | 24 (70.6) | 11 (68.8) | 1.000 |
| Neurological manifestations | 5 (14.7) | 3 (18.8) | 0.699 |
| Serositis | 4 (11.8) | 4 (25) | 0.249 |
| Skin manifestations | 18 (52.9) | 9 (56.3) | 1.000 |
| Edema | 3 (8.8) | 4 (25) | 0.190 |
| Family history of Rheumatological diseases | 6 (17.6) | 1 (6.3) | 0.406 |
| At the time of study | |||
| Consumed C3 | 18 (52.9) | 0 (0) | 0.000* |
| Consumed C4 | 17 (50) | 0 (0) | 0.000* |
| Albuminuria | 12 (35.3) | 4 (25) | 0.533 |
Comparison Between Active and Inactive Cases According to Different Manifestation and Investigationsa
As regards treatment, there was no significant value between the two groups, except that the doses of steroids were significantly higher in active cases than in inactive cases (13.24 ± 10.22 in active group vs. 6.17 ± 3.52 in inactive group) (P = 0.002).
We classified active cases (34 cases) according to degree of activity into two groups: group (I) with mild activity which represents 16 cases and group (II) with moderate or severe activity which represents 18 cases.
Duration of disease in group I ranged between 4 and 168 months with a mean of 53.19 ± 58.11 while duration of disease in group II ranged between 2 and 84 months with a mean of 47.89 ± 23.79 with no significant value (P = 0.476).
Comparison according to clinical manifestations and investigations revealed that hypertension was significantly more presented in group II than in group I (P = 0.046) (Table 6).
| Variables | Group I (Mild Activity) (N = 16) | Group II (Moderate or Severe Activity) (N = 18) | P Value |
|---|---|---|---|
| Hypertension | 0 (0) | 5 (27.8) | 0.046* |
| Malar rash | 5 (31.3) | 8 (44.4) | 0.497 |
| Photosensitivity | 4 (25) | 9 (50) | 0.172 |
| Lupus nephritis | 11 (68.8) | 8 (44.4) | 0.185 |
| ANA ve+ | 11 (68.8) | 17 (94.4) | 0.078 |
| +ve Anti-DNA | 11 (68.8) | 8 (44.4) | 0.497 |
| Consumed C3 | 10 (62.5) | 6 (33.3) | 0.168 |
| Consumed C4 | 10 (62.5) | 7 (38.9) | 0.303 |
| Albuminuria | 5 (31.3) | 7 (38.9) | 0.729 |
| IL-37 | 19.00 ± 28.48 | 23.56 ± 40.35 | 0.23 |
| TLC, thousands/cmm | 7.66 ± 3.34 | 6.733 ± 30 | 0.227 |
| Platelet count, thousands/cmm | 314.50 ± 88.27 | 278.78 ± 102.13 | 0.157 |
Comparison Between Degree of Activity According to Clinical Manifestations and Investigationsa
The level of IL-37 was higher in group II than in group I, with no statistically significant difference (P = 0.230).
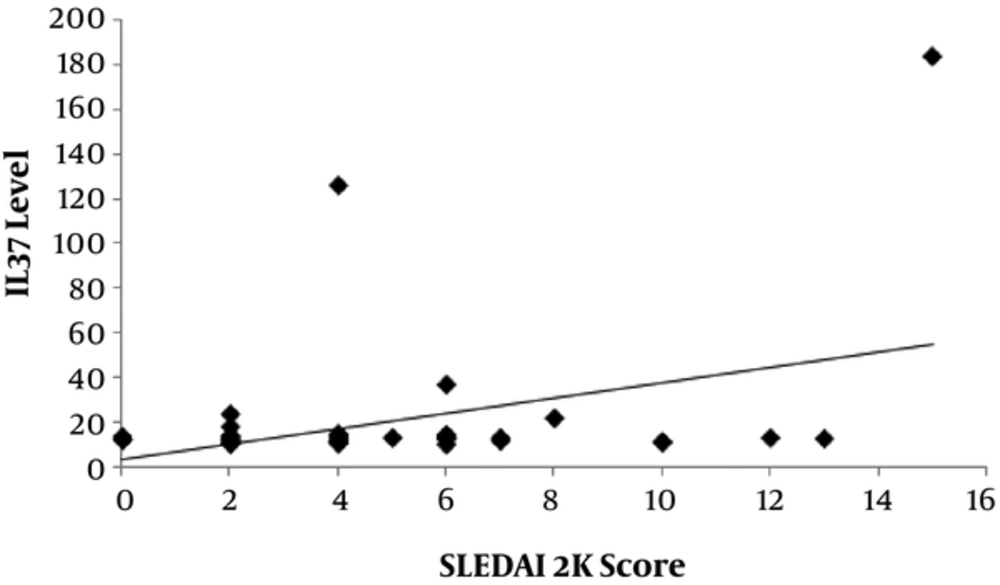
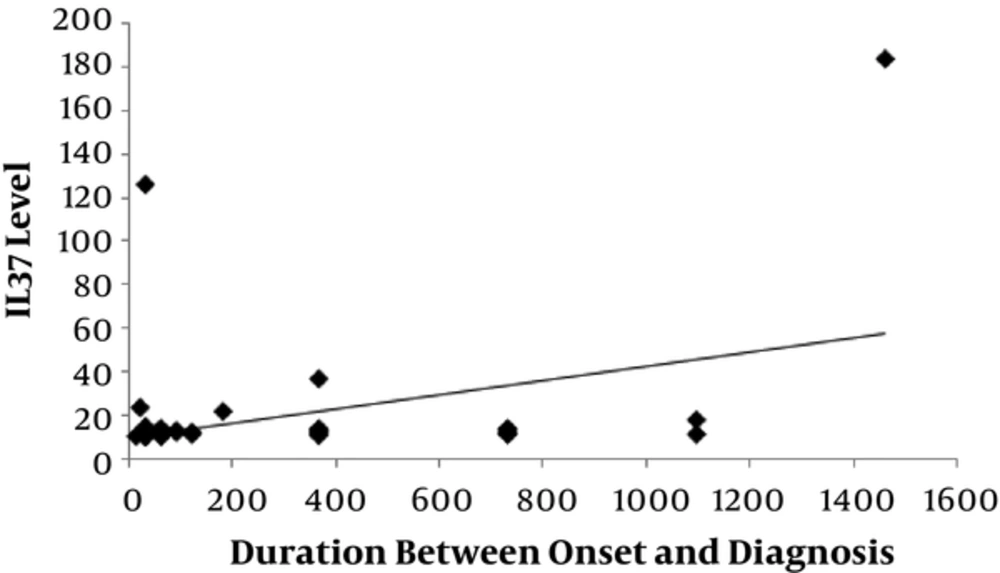
There was a significantly positive correlation between level of IL-37 and SLEDAI-2K score (P = 0.006) with r = 0.383 as shown in Figure 2 and also significant positive correlation between level of IL-37 and the duration between onset and diagnosis of disease (P = 0.007) with r = 0.378 as shown in Figure 3).
4. Discussion
Systemic lupus erythematosus (SLE) is a chronic, inflammatory, autoimmune disorder with multi-organ involvement, characterized by autoantibody production and immune complex deposition (10).
IL-37 is considered as an anti-inflammatory cytokine, which suppresses innate immune response (11).
In our study, percentage of lupus nephritis was 64%. This finding was relatively close to the result of a study done by Pisoni et al. (12), 2015, where percentage of lupus nephritis was 66.7% and higher than a study done by Youssef et al. (13), 2015, where percentage of lupus nephritis was 50%.
In our study, percentage of arthritis was 70%. Our finding was close to the result of a study done by Song et al. (1), 2013, where percentage of arthritis was 73.3% and higher than a study done by Abdwani et al. (14), 2014, where percentage of arthritis was 62.96%.
Levels of IL-37 in the SLE cases were higher than in the controls. The IL-37 levels are also compared between cases and controls using non-parametric tests. We found that the median of IL-37 in cases was 11.9 with the interquartile range (IQR) 11-13.2, while the median of IL-37 in controls was 11 with the IQR 10.63-12.3, with P value of 0.028 which was statistically significant.
In a study by Ye et al. (6), 2014, , the levels of IL-37 in SLE cases were higher than in controls with statistically significant value (P = 0.0009).
Percentages of positive ANA and positive ant-dsDNA were 80% and 40% respectively. Our findings were lower than that of Lotfy et al. (15), 2015, where percentages of positive ANA and positive ant-dsDNA were 95% and 50% respectively.
In our study, the SLEDAI-2K score ranged between 0 and 15 (4.60 ± 3.220), which was relatively close to the result of a study done by Ye et al. (6), 2014, where the SLEDAI-2K score ranged between 2 and 14 and lower than that of Song et al. (1), 2013, where the SLEDAI-2K score was 15.33 ± 3.79.
In our study, we next investigated whether IL-37 was related to disease activity. We found that levels of IL-37 in active cases were higher than in inactive cases, although without a statistically significant value (P = 0.580). Our finding came in agreement with Ye et al. (6), 2014, where the levels of IL-37 in active cases with SLE were higher than in inactive cases.
Comparison between active and inactive cases revealed statistically significant difference between two groups only in consumed C3 and C4 as we found it more frequent in active group than in inactive one. Also steroid doses were higher in active than in inactive cases with statistically significant value (P = 0.002).
Our study revealed that levels of IL-37 were positively correlated with SLEDAI-2K score which came in agreement with a study done by Song et al. (1), 2013. We also found that levels of IL-37 were positively correlated with duration between onset and diagnosis of the disease.
There was no significant correlation between level of IL-37 and lupus nephritis. Our finding came in contrast with the study by Ye et al. (6), 2014, where serum IL-37 levels were significantly higher in lupus nephritis patients.
We then subdivided active SLE cases according SLEDAI-2K score. We found that level of IL-37 level in mild activity was lower than in moderate or severe activity, however it did not reach a statistically significant value (P = 0.230).
Comparison between mild activity group and moderate or severe activity group according to clinical manifestations revealed statistically significant difference between two groups only in presence of hypertension (P = 0.046) as we found it more frequent in moderate or severe activity group than in mild activity group.
A major limitation of this study was the small sample size and lack of follow up of SLE patients.
4.1. Conclusion
The present study demonstrated that level of IL-37 was higher in our SLE patients than in healthy controls and it was higher in active cases than in inactive cases. Level of IL-37 is positively correlated with the duration between onset and diagnosis of disease and SLEDAI-2K score.
We recommend assessing IL-37 before and after using treatment to detect the role of treatment in changing the level of IL-37.