1. Background
Early childhood caries (ECC) is an aggressive form of dental caries, which begins with the teeth eruption and progresses rapidly (1). Biologically, ECC is an infectious process, in which Streptococcus mutans is the primary pathogen degrading sugar and developing caries (2). ECC is defined as the presence of more than one decayed, missing, or filled tooth in children aged below 71 months (3).
As a biologic fluid, saliva has a high potential for dental research (4). Saliva sampling is non-invasive and easily-collected and can be repeated; hence, it is an ideal diagnostic fluid for screening and controlling many diseases. Since the teeth are in constant contact with saliva, the properties of saliva play a critical role in developing caries. Moreover, saliva is the first defense line against free radicals in the body (5).
Reactive oxygen species (ROS) are the derivatives of oxygen metabolism in all biological systems; hence, the ROS production is the central part of human metabolism under different physiological conditions. Oxidative stress occurs when the ROS level exceeds the significance level. Oxidative stress results from the imbalance of ROS, free radicals, and the antioxidant system and acts as an essential etiological factor in many oral inflammatory pathologies (6).
Lipid peroxidation (LPO) is the primary outcome of the ROS-induced tissue damage. LPO begins with the reaction of ROS with unsaturated fatty acids or lipoproteins of the membrane, altering the structural integrity and function of the cell membrane (7). Malondialdehyde (MDA) is one of the main and best products to evaluate unsaturated fatty acid peroxidation reactions (5, 8). MDA is increased during the inflammatory processes and can damage the structure and function of cell membranes in the body if the body's reduction system does not neutralize it. It is produced by ROS and is used as a biomarker of oxidative stress (9).
The biomarkers of oxidative stress increase in various diseases, including periodontal diseases and dental caries (10, 11). In a study on the salivary MDA in children with and without ECC, Subramanyam et al. reported an increase in the MDA level in the ECC group (12). Sarode et al. (13) examined the salivary MDA in individuals with caries and observed that the MDA levels were higher in those with caries than in caries-free persons. However, in some studies (e.g., Silva et al. (14)), the salivary MDA levels were decreased in patients with caries. These contradictory findings suggest the ambiguous relationship between the biomarkers of oxidative stress and diseases such as dental caries.
Although many studies have addressed ECC and its relationship with salivary compounds, few studies have dealt with the salivary MDA in children with ECC and between the two genders. Moreover, given the different findings on the MDA levels in different pathologies, further studies are required to confirm the relationship between oxidative stress and pathologies.
2. Objectives
The present study aimed to investigate the salivary MDA levels in children with ECC and detect the role of MDA in the prevention and early diagnosis of individuals susceptible to caries.
3. Methods
3.1. Sampling
The present study was confirmed and approved by the Ethics Committee of the Isfahan University of Medical Sciences (Code: IR.MUI.RESEARCH.REC.1398.163). Informed consent was obtained from the parents to include their children in the study. In this study, 42 children with ECC (30 boys and 12 girls) and 42 caries-free children (24 boys and 18 girls), aged 4 - 6 years, were randomly selected from the kindergartens of four socio-economically different districts of Isfahan, Iran. Individuals with systemic, acute, or chronic disease, who were taking antibiotics or anti-inflammatory drugs or multivitamin supplements during the past three months were excluded from the study. Further, children with no sign of dental hypoplasia or fluorosis, decreased salivary flow, salivary gland disorders, and oral lesions associated with bleeding were not also included in the study (2, 12, 14). The participants were assigned into the ECC and CF groups based on visual examinations by a pediatric dentist. ECC is operationally defined as the presence of more than one decayed, missing, or filled primary tooth in children aged six years or below
3.2. Clinical Examination
The single examiner trained by an experienced pediatric dentist performed all the visual examinations. About 10% of the participants were re-examined to determine the intra-examiner reliability. Acceptable intra-examiner reliability was established for dmft > 90%. Children's examination was performed in a supine position, using a mouth mirror, explorer, and headlight with no radiography. The World Health Organization’s (WHO) recommendations were also considered to record the caries status (15).
3.3. Saliva Collection
Unstimulated saliva is a mixture of secretions, which resembles plasma in composition and enters the mouth with no exogenous stimuli. The unstimulated saliva sampling method is often preferred since the dilution of analytes is minimized in this method. Unstimulated saliva is less dependent on flow rate and pH; however, it depends on the daily basal salivary flow rate in the oral cavity (16).
The unstimulated saliva samples were collected from the participants using the spitting method in 10 minutes. The samples were collected one hour after routine brushing with water and fluoride-free products. Sampling of children was performed in a sitting upright position with the head slightly tilted forward. In the present study, saliva samples were collected in the morning (8 - 10 a.m.), and the participants were asked to be on fast the night before sampling to reduce daily changes. The collected samples were kept in sterile air-tight tubes at -20°C.
3.4. MDA Measurement
The salivary MDA was analyzed using a commercial kit (ZellBio GmbH, Germany), according to the manufacturer's instructions. This kit is a simple, reproducible, and standardized tool to analyze lipid peroxidation in biological samples. According to the instructions, all reagents were equilibrated at room temperature. The samples well-shacked for homogenization, and 50 μL standards/samples were added to relevant test tubes regarding their name. In the next step, 50 μL R4 and then 1mL ready Chromogenic solution were added. The resulting solution was kept in the boiling water bath (90 - 100°C) for 1 hour. When the solution cooled down, the samples were centrifuged at 3000 - 4000 rpm. Moreover, 200 μL of pink color supernatant was pipette into the microplate. The absorbance was obtained with a microplate reader/ELISA reader at 535 nm. The MDA level was evacuated in unknown samples based on the standard curve, which is drowned using the absorbance of standard points.
3.5. Statistical Analysis
The data were analyzed with SPSS software version 22. The children in the control and ECC groups were compared for the salivary MDA level using an independent student's t-test. The same test was also used to determine the relationship between gender and the MDA level. Pearson correlation coefficient was used to evaluate the relationship between the caries rate and the MDA level at P < 0.05. The results for the MDA level are reported as micromole per liter.
4. Results
Eighty-two children, aged 4 - 6 years, were recruited in this case-control study, of whom 42 children with ECC (30 boys and 12 girls) and 42 caries-free children (24 boys and 18 girls) were assigned into the experimental and control groups, respectively.
4.1. MDA Levels in ECC and Control Groups
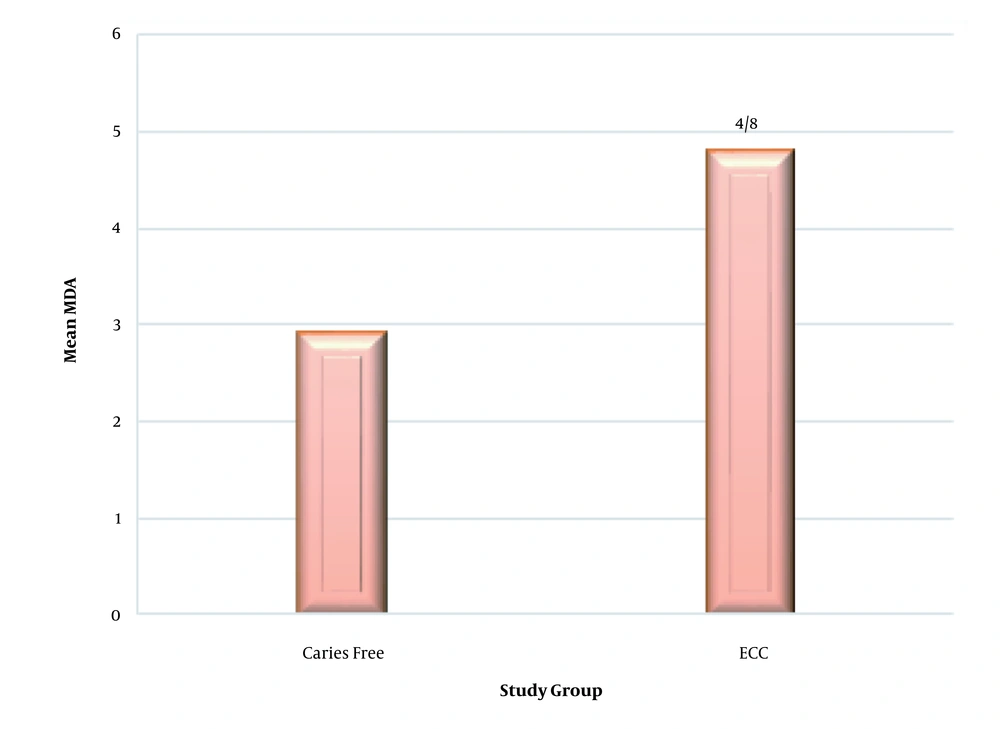
The experimental and control groups were compared in terms of the salivary MDA. The mean salivary MDA was significantly higher in the ECC group (4.8 ± 0.6) than the caries-free group (2.9 ± 0.5) (P = 0.01) (Figure 1).
4.2. MDA and dmft Scores
The mean dmft index was 7.02 ± 2.84 in the ECC group (7.92 ± 1.78 and 6.67 ± 3.12 in boys and girls, respectively). The Pearson correlation coefficient test showed no significant relationship between the salivary MDA and dmft levels in the ECC group (P = 0.36) (Table 1).
| Variable | dmft | |
|---|---|---|
| R | P-Value a | |
| Mean MDA level (μmol/L) | 0.146 | 0.36 |
Pearson Correlation Coefficient Between Salivary MDA and dmft in ECC Group
4.3. MDA and Gender
The mean salivary MDA was not significantly different between the boys and girls in the control (P = 0.30) and ECC (P = 0.44) groups. In other words, there was no significant correlation between the salivary MDA levels and gender (Table 2).
5. Discussion
ECC is of great concern due to its adverse effects on children's life, including increased treatment costs, increased risk of permanent tooth caries, delayed growth and development, reduced learning ability and natural activities, and reduced physical health and quality of life (17-19). Since oxidative stress reactions result in various pathologies, measuring the biomarkers of these reactions, including MDA, facilitates the early diagnosis of diseases.
In this study, the salivary MDA level, as the primary biomarker of oxidative stress reactions, was measured in the ECC children. The results revealed that the mean salivary MDA levels were 4.8 (5.1 in girls and 4.1 in boys) in the ECC group and 2.9 (2.4 in girls and 3.4 in boys) in the caries-free group. Accordingly, the mean salivary MDA level was higher in the ECC group than the caries-free group. Many studies have reported similar results. For example, Subramanyam et al. (12), Ahmadi-Motamayel et al. (20), and Banihashemrad et al. (21) also reported higher MDA levels in the ECC group than the caries-free group, indicating the elevated levels of oxidative stress reactions in the dental caries process. Further, Canakci et al. (22) showed that the MDA levels increased in areas with inflammation and infection. Accordingly, the MDA level is associated with the spread of infection and inflammation. Furthermore, Southward (23) reported the elevated levels of oxidative stress reactions during the destruction of dental hard tissue. Dental caries trigger the activity of phagocytes, thereby promoting the production of free radicals. In this regard, the lipid peroxidation and MDA levels also increase (24).
In contrast, Silva et al. (14) reported the lower salivary MDA level in children with ECC than the caries-free children. This difference in findings can be due to heterogeneous sampling conditions. Since the study was conducted in Brazil, participants’ racial and genetic differences may have aroused inconsistencies in results. Moreover, in their study, there was a two-hour interval between the last meal and sampling. However, like most physio-pathological tests, 8-hour fasting before sampling was considered in the present study to eliminate the intervening effects of food on the study variable (25). Foods containing flavonoids and antioxidants such as albumin and ascorbic acid (vitamin C) increase the antioxidants (26). On the other hand, during the digestion of certain foods such as meat, which contains arginine amino acid, the oxidative stress reactions and MDA increase subsequently (27). In this regard, with an 8-hour fasting interval, the impact of food on the MDA level was eliminated. Since circadian changes influence the composition and amount of saliva secretion, the saliva is more concentrated and has the highest compounds in the early morning. In other words, morning is ideal to collect saliva samples (28). Moreover, the contradictory results in Silva et al.’s study may have caused by the participants’ different age ranges (0 - 3 years). In a similar vein, Patel and Pujara (29) and Hegde et al. (30) reported an increase in the biomarkers of oxidative stress by increasing age.
From another perspective, the contradictory results of the studies could be due to different sampling times at different stages of the disease as the antioxidant capacity increases in the acute stage of the disease. However, as the disease progresses, the antioxidants emerge in the oxidative stress reactions and reduce the body's defense mechanism (31).
Given the limited number of the samples, it was impossible to match the samples in terms of gender in the ECC and caries-free groups to detect the correlation between gender and MDA. However, the results of this study indicated that the mean MDA level was significantly higher in the ECC group than the caries-free group. Furthermore, there was no significant difference between the girls and boys in the ECC and caries-free groups in terms of the mean salivary MDA. In other words, gender seems to have no effect on the salivary MDA in 4 - 6-year-old children. However, Ahmadi-Motamayel et al. (20) showed a significant difference between MDA levels regarding the participants’ genders.
In the present study, the mean rate of dmft in the ECC group was 7.02 ± 2.84; however, there was no significant relationship between dmft and MDA in the ECC group. In other words, by increasing the number of carious teeth, the salivary MDA level did not change significantly. The inflammation caused by dental infection increases the salivary MDA level; hence, the presence or absence of dental infection is more prominent in altering the MDA level than the number of infected teeth. Moreover, as caries progresses, the antioxidants emerge in the oxidative stress reactions and reduce the body's defense mechanism (31).
The same finding was also reported by Ozturk et al. (32) and Subramanyam et al. (12). However, Tsai et al. (33) and Sculley et al. (34) indicated that individuals with poor oral hygiene had higher lipid peroxidation (LPO) levels.
Early childhood caries is a severe challenge to pediatric dentists. The early detection of ECC risk factors can help the practitioner prevent the caries process in children. MDA increases secondary to the increased inflammation caused by dental infection, and the assessment of MDA is helpful but time-consuming. In this regard, antioxidants can be administered to neutralize free radicals caused by oxidative stress and reduce bacterial infections (35, 36).
Oxidative stress reactions increase in different diseases (37). To eliminate the effect of other variables increasing such reactions, individuals with systemic diseases were excluded from the study as such the likelihood of bias decreased. Furthermore, taking supplements with flavonoids and antioxidants and anti-inflammatory compounds increases the number of antioxidants and shifts the balance toward increased antioxidants.
The study findings need to be considered with regard to the research limitations. Since this study was cross-sectional, we failed to provide any causal explanation; hence, the findings must be explained with caution. Another limitation of this study was that its relatively small sample size. Further studies are required to be conducted with a larger sample size. Future studies are also recommended consider the oral hygiene level and its relationship with the MDA level.
5.1. Conclusions
According to the study results, there was an association between ECC and MDA, one of the products of oxidative stress reactions. MDA increases secondary to the increased inflammation caused by dental infection as such the MDA assessment would be of benefit. The significant association between the salivary MDA and ECC indicated that saliva could be used as an important diagnostic sample for susceptibility to dental caries. However, further studies need to be conducted in this regard.