1. Background
Congenital diaphragmatic hernia (CDH) occurs in 3 to 3.6 per 10,000 live births, with about 85% of affected neonates having left-sided hernias. CDH is caused by the incomplete closure of the fetal diaphragm muscle during embryonic development, allowing the abdominal viscera to herniate into the thoracic cavity and compress the heart and the lungs. CDH may present in the first few hours of life with pulmonary artery hypertension (PAH), which rapidly progresses and leads to respiratory distress and death. The overall survival of CDH after surgery can reach up to 80% (1). However, the optimal timing of surgery and the choice between emergency surgery and delayed repair remain unclear (2).
In this regard, Rozmiarek et al. (3) reported that CDH repair should be based on the patient’s clinical improvement, that is, a normal mean artery pressure according to gestational age; an estimated pulmonary artery pressure lower than whole-body blood pressure; preductal oxygen saturation (SpO2) between 85 and 95%, with an inspired oxygen concentration below 50%; lactic acid concentration < 3 mmol/L; and urine volume > 2 mL/kg/h (1, 4). These conditions reduce the risk of anesthesia and increase the postoperative survival (3, 5). Newborns with mild-to-moderate CDH, based on the lung-to-head ratio (LHR ≥ 1.0), are more likely to meet these physiological standards compared to severe cases (LHR < 1.0) (6). Therefore, one of the main reasons why the optimal timing of surgery remains unclear is that there is no separate study on mild-to-moderate and severe cases, while the optimal timing of surgery for these cases should be different.
2. Objectives
The present study aimed to determine the optimal timing of surgery for left-sided mild-to-moderate CDH, based on LHR.
3. Methods
3.1. Patients
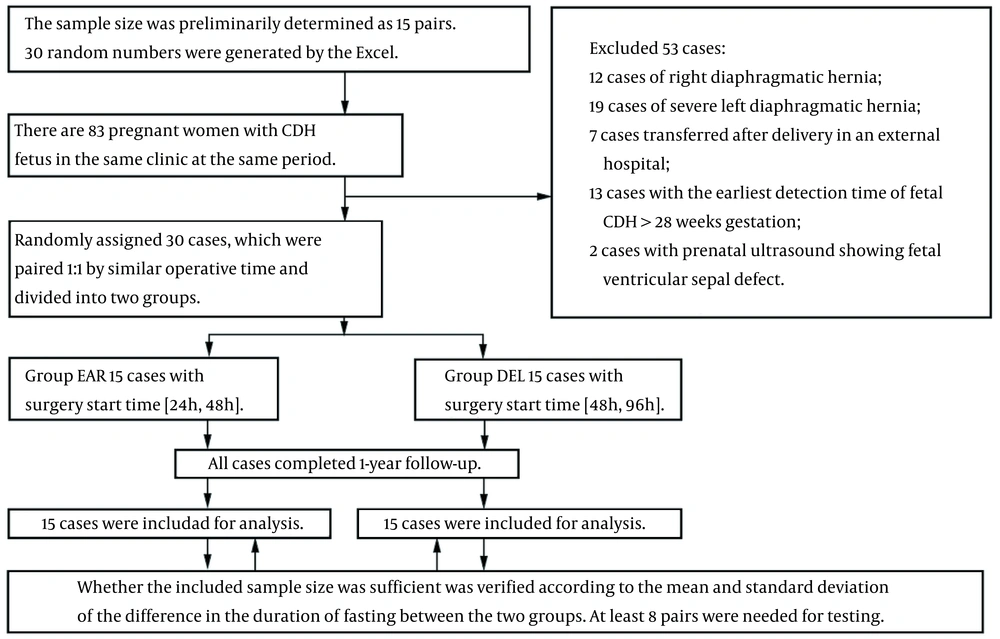
This clinical trial, including 30 newborns with left-sided mild-to-moderate CDH, was conducted from August 15, 2017 to May 24, 2019 at the Department of Obstetrics and the neonatal intensive care unit (NICU) of the Department of Pediatric Thoracic Surgery in our hospital. The inclusion criteria were as follows: (1) left-sided CDH diagnosed by prenatal ultrasound; and (2) LHR > 1.0 based on level III obstetric ultrasound during 24 - 28 weeks of pregnancy (1, 3, 4). On the other hand, the exclusion criteria were other cardiac malformations, such as incomplete closure of the patent ductus arteriosus (PDA) and patent foramen ovale (PFO), detected by prenatal ultrasound.
Preparation for surgery
The patients were transferred to the NICU within ten minutes after delivery and underwent mechanical ventilation during continuous sedation with midazolam (0.1 mg/kg/h) and fentanyl (3 µg/kg/h). SpO2 was maintained at 80 - 95%. Mezlocillin and sulbactam were also injected (75 mg/kg/q8h) to prevent infection. Administration of vasoactive agents and fluid infusion was performed to maintain a normal arterial pressure, according to the patient’s gestational age. Meanwhile, heart rate, preductal/postductal oxygen saturation, and arterial blood gas (ABG) were monitored closely.
Chest and abdominal X-ray scans were acquired, and echocardiography was scheduled within the first 24 hours after birth. Moreover, PAH in the affected neonates was categorized into three levels: no/mild PAH, pulmonary hypertension (PH) < 2/3 of the whole-body systolic blood pressure; moderate, whole-body systolic blood pressure > 2/3 of the whole-body systolic blood pressure; and severe, PH ≥ whole-body systolic blood pressure (7, 8).
3.2. Surgical Procedures
All surgeries were performed via thoracoscopy. After the patient was placed in the right lateral position, the intercostal location of the subscapular angle was selected to make a thoracoscopic observation hole and to perform artificial carbon dioxide pneumothorax. The operating ports were made in the seventh intercostal space of the posterior axillary line and the sixth intercostal space of the midaxillary line. The positions of the two operating ports were adjusted according to the diaphragm defect.
Using manipulation forceps, the hernia contents, including the intestines, spleen, and hernia sac, were repositioned to the abdominal cavity. Next, the defective diaphragm was sutured intermittently with 4 - 0 Prolene sutures. A drainage tube, inserted into the thoracic cavity during surgery, was retained for closed drainage to avoid lung compression caused by postoperative pleural effusion and to detect potential postoperative hemothorax, pneumothorax, or chylothorax. No patches were used in any of the surgeries. No extracorporeal membrane oxygenation (ECMO) was used before, during, or after surgery.
3.3. Postoperative Management
Postoperative echocardiography was performed within 72 hours after surgery. In case of right-to-left shunting in the arterial duct or the oval foramen, an oxygenation index > 20, or a difference in preductal/postductal oxygen saturation exceeding 10%, inhalation of nitrogen monoxide and intravenous injection of prostaglandin E1 were required. Antibiotic treatment was discontinued if inflammation was properly managed using appropriate tests. As soon as 20 mL/d (or less) of fluid was obtained from the closed drainage, the drainage tube was removed from the thoracic cavity.
Mechanical ventilation was discontinued with chest X-ray, indicating controlled pneumonia. Meanwhile, the mean airway pressure (MAP) was < 8 mbar, the recurrence rate reduced to 30 times/min, and the inspired oxygen concentration reached 30% or less. The ABG reached the target range (PO2, 50 ~ 70 mmHg; PCO2, 40 ~ 50 mmHg; and pH, 7.25 ~ 7.40), and the neonates showed no significant breathing efforts. The tracheal tube was then removed and changed to nasal continuous positive airway pressure (NCPAP) (9). Withdrawal of mechanical ventilation was followed by nasal feeding with an enteral feeding device from 10 mL/q3h to 30 mL/q3h. A feeding bottle was then used, and the patient was discharged after the nutritional feed reached 100 mL/kg/d.
3.4. Primary and Secondary Endpoints
The primary endpoint was the duration of fasting, which included the duration of preoperative mechanical ventilation, surgery duration, and duration of postoperative mechanical ventilation; it represented the duration of treatment. The secondary endpoints were surgery duration, duration of antibiotic use, duration of postoperative mechanical ventilation, incidence of postoperative moderate-to-severe PAH, postoperative mortality, recurrence rate during follow-up, hospital stay, and hospitalization costs.
3.5. Follow-up
The patients were instructed to return to the clinic at seven days, one month, and six months after discharge. The following examinations were performed for these patients: (1) chest and abdominal radiography to assess pneumonia or diaphragmatic hernia recurrence; and (2) blood routine and high-sensitivity C-reactive protein (CRP) tests to assess inflammation. A telephone follow-up was performed one year after discharge to assess the chest radiograph results performed at the local hospital.
3.6. Sample Size Calculation
The sample size was determined based on the mean and standard deviation (SD) of the difference in the duration of fasting between the two groups. The two patient groups were paired 1: 1, based on a similar surgery time. Paired measurements were used as the measurement data; the data were found to be normally distributed (W = 0.919, P = 0.213, δ = -31.357, σ = 21.500) (Table 1), based on paired t-test. The sample size was calculated using the following formula (10):
Abbreviation: DF, duration of fasting (h).
a Values are expressed as mean ± SD unless otherwise indicated.
b Shapiro-Wilk test.
c Paired-samples t test.
Based on the findings, at least eight pairs were needed for testing.
3.7. Statistical Methods
SPSS version 22.0 was used for statistical analysis. Continuous variables were expressed as mean ± SD, and paired t-test was used for intergroup comparisons. Categorical variables were expressed as percentage, and chi-square test was performed for comparisons. Ranked data were also expressed as mean ± SD, and Wilcoxon signed-rank test was carried out for comparisons. P < 0.05 was considered statistically significant.
4. Results
4.1. Study Flowchart
A total of 83 pregnant women with CDH of the fetus were enrolled in this study. However, 53 cases were excluded. The remaining 30 patients were randomized to emergency (EAR) and delayed (DEL) groups (n = 15 per group). The two patient groups were paired 1: 1, based on a similar surgery time (Figure 1).
4.2. Baseline Patient Characteristics
According to LHR, there were four mild and 11 moderate CDH cases in each group. The EAR group included ten natural delivery and five C-section cases. In the DEL group, there were 11 patients born naturally and four patients born via C-section. Based on the echocardiographic assessment within 24 hours after birth, mild PAH was found in five patients in the EAR group, while ten patients were PAH-free. In the DEL group, there were 12 patients without PAH, and three patients had mild PAH. The hernia sac-free/hernia sac ratios in the EAR and DEL groups were also 10: 5 and 14: 1, respectively.
The two groups showed no significant differences in terms of the measured LHR, LHR-based mild/moderate CDH ratio, days of pregnancy, natural delivery/C-section ratio, male/female ratio, birth weight, one-minute Apgar score, preoperative PAH-free/mild PAH ratio, and hernia sac-free/hernia sac ratio (P > 0.05 for all). However, age at surgery (duration of preoperative mechanical ventilation) was significantly different between the two groups (P = 0.001) (Table 2).
| Variables | EAR | DEL | Mean ± SD | Normality Test of Differential Value (W, P) | Statistic | P |
|---|---|---|---|---|---|---|
| LHR | 1.562 ± 0.287 | 1.697 ± 0.444 | -0.135 ± 0.506 | (0.979, 0.964) b | -1.034 c | 0.319 |
| M/M | 11/15 | 11/15 | - | - | 0.001 d | 1.000 |
| Ds | 273.267 ± 7.564 | 276.667 ± 9.431 | -3.400 ± 10.425 | (0.913, 0.151) b | -1.263 c | 0.227 |
| N/C | 10/15 | 11/15 | - | - | 0.159 d | 0.690 |
| M/F | 3/15 | 7/15 | - | - | 2.400 d | 0.121 |
| Wt | 3.151 ± 0.419 | 3.183 ± 0.557 | -0.032 ± 0.675 | (0.936, 0.331) b | -0.184 c | 0.857 |
| Ap1 | 8.400 ± 0.632 | 8.333 ± 0.617 | - | - | -0.277 e | 0.782 |
| F/M | 10/15 | 12/15 | - | - | 0.682 d | 0.409 |
| Age | 37.067 ± 5.898 | 69.600 ± 9.448 | -32.533 ± 11.307 | (0.968, 0.826) b | -11.144 c | 0.001 |
| F/S | 10/15 | 14/15 | - | - | 3.333 d | 0.068 |
Abbreviations: LHR, lung-to-head ratio; M/M, LHR-based mild/moderate CDH ratio; Ds, days of pregnancy; N/C, natural delivery/C-section ratio; M/F, male/female ratio; Wt, birth weight (kg); Ap1, one-minute Apgar score; F/M, preoperative PAH-free/mild PAH ratio; Age, age at surgery (h); F/S, hernia sac-free/hernia sac ratio.
a Values are expressed as mean ± SD unless otherwise indicated.
b Shapiro-Wilk test.
c Paired-samples t test.
d Chi-square test.
e Wilcoxon signed rank test (2-tailed).
4.3. Primary Endpoint
The duration of fasting showed significant differences between the EAR and DEL groups (199.429 ± 15.873 vs. 230.786 ± 17.348 hours; P = 0.001) (Table 1).
4.4. Secondary Endpoints
The surgery duration, duration of postoperative mechanical ventilation, incidence of postoperative moderate-to-severe PAH, postoperative mortality, and hospitalization costs were similar between the two groups (P > 0.05 for all). However, antibiotic use (P = 0.004) and hospital stay (P = 0.032) showed significant differences between the two groups (Table 3).
| Variables | EAR | DEL | Differential Value | Normality Test of Differential Value (W, P) | Statistic | P |
|---|---|---|---|---|---|---|
| SD | 107.533 ± 12.287 | 107.800 ± 8.170 | -0.267 ± 12.584 | (0.981, 0.976) b | -0.082 c | 0.936 |
| AN | 269.533 ± 27.518 | 304.733 ± 33.518 | -35.200 ± 39.232 | (0.927, 0.250) b | -3.475 c | 0.004 |
| DPV | 159.333 ± 15.573 | 156.867 ± 12.438 | 2.467 ± 18.647 | (0.942, 0.414) b | 0.512 c | 0.616 |
| PPR | 2/15 | 1/15 | - | - | 0.370 d | 0.543 |
| PM | 1/15 | 1/15 | - | - | 0.001 d | 1.000 |
| RE | 0 | 0 | 0 | - | - | - |
| HS | 15.067 ± 2.219 | 17.067 ± 2.492 | -2.000 ± 3.251 | (0.921, 0.202) b | -2.382 c | 0.032 |
| HC | 50162 ± 8847 | 51494 ± 8378 | -1332 ± 12094 | (0.901, 0.097) b | -0.427 c | 0.676 |
Abbreviations: SD, surgery duration (min); AN, antibiotic use (h); DPV, duration of postoperative mechanical ventilation (h); PPR, incidence of postoperative moderate-to-severe PAH; PM, postoperative mortality; RE, recurrence rate during follow-ups; HS, hospital stay (day); HC, hospitalization costs (CNY).
a Values are expressed as mean ± SD unless otherwise indicated.
b Shapiro-Wilk test.
c Paired-samples t test.
d Chi-square test.
There were one moderate and one severe case of postoperative PAH in the EAR group. In the DEL group, no moderate postoperative PAH was found, while one patient had severe postoperative PAH. One case of postoperative death due to severe PAH was reported in each group. These patients died at eight and nine days following CDH repair; therefore, the two groups had a similar postoperative mortality rate. The incidence rates of postoperative moderate-to-severe PAH, postoperative mortality, and recurrence during follow-up were similar between the two groups (P > 0.05 for all). No intraoperative mortality or in-hospital recurrence was reported. After discharge from the hospital, there was no loss to follow-up in either of the groups. During a 12-month follow-up, no case of CDH recurrence was observed, and no patient died during the follow-up (Table 3).
4.5. Adverse Effects
The postoperative drainage volume, duration of postoperative drainage, incidence of postoperative gastroesophageal reflux disease (GERD), postoperative pulmonary infection rate, and incision infection rate were similar between the two groups (P > 0.05 for all) (Table 4).
| Variables | EAR | DEL | Differential Value | Normality Test of Differential Value (W, P) | Statistic | P |
|---|---|---|---|---|---|---|
| VOL | 181.000 ± 163.250 | 201.333 ± 154.220 | -20.333 ± 201.711 | (0.925, 0.227) b | -0.390 c | 0.702 |
| DD | 4.600 ± 3.043 | 5.467 ± 3.182 | -0.867 ± 4.172 | (0.916, 0.168) b | -0.804 c | 0.435 |
| PGR | 2/15 | 4/15 | -- | -- | 0.833 d | 0.361 |
| PIR | 3/15 | 7/15 | -- | -- | 2.400 d | 0.121 |
| IIR | 2/15 | 2/15 | -- | -- | 0.001 d | 1.000 |
Abbreviations: VOL, postoperative drainage volume (mL); DD, duration of postoperative drainage (day); PGR, incidence of postoperative gastroesophageal reflux disease (GERD); PIR, postoperative pulmonary infection rate; IIR, incision infection rate.
a Values are expressed as mean ± SD unless otherwise indicated.
b Shapiro-Wilk test.
c Paired-samples t test.
d Chi-square test.
5. Discussion
Physiologically, PAH is characterized by the excessive proliferation of pulmonary artery smooth muscle cells (PASMCs), leading to thickened tunica adventitia and tunica media and reduced elasticity of pulmonary arteries. Besides, increased pulmonary vascular resistance triggers right-to-left shunting, resulting in hypoxemia and a difference in preductal/postductal oxygen saturation. In this trial, echocardiography within 24 hours after birth showed no mild or moderate-severe PH, which is consistent with prenatal ultrasound LHR at 24 - 28 weeks of pregnancy. The pulmonary arterial pressure changed in only a few patients, and these changes were not significantly different between the two groups. Two children died of severe PH after surgery.
It is known that children's LHR, pulmonary artery pressure, and prognosis are basically similar (11). Studies have shown that in PAH, as PH declines over time, delayed surgery poses a lower risk of PAH (12). However, since delayed repair refers to the prolonged use of mechanical ventilation, there is a higher risk of preoperative and perioperative infections and other complications, such as ventilator-associated infection (13). Moreover, the period of antibiotic use is extended, which in turn increases the risk of drug resistance and fungal infection (14).
When respiratory and circulatory functions are stabilized, the sooner the surgery is completed, the more the patient can benefit from it. First, the lungs can be freed from compression caused by the abdominal viscera, which facilitates the re-expansion of the lungs, relieves acid respiration and PAH, and accelerates improvements in pulmonary function (15). When the lungs are compressed by the hernia content, there is a chance of lung consolidation and dead space, leading to retention of carbon dioxide and acid respiration. Ultimately, blood acidification results in pulmonary artery spasm and aggravates PAH (16).
Methylmalonic acidemia (MMA) is characterized by persistent PAH in neonates (17). Meanwhile, pulmonary hypoplasia in CDH is often associated with lung overexpansion and poor tolerance to elevated expansion pressure. After CDH repair, a relatively low expansion pressure may improve the respiratory mechanics in neonates with mild-to-moderate CDH (15). Second, since PAH not only leads to right ventricular volume overload, but also causes a left ventricular dysfunction, early repair can reduce the pressure from the hernia contents on the heart, promote left ventricular function recovery, and accelerate the overall rehabilitation (4). Third, early repair reduces the risk of complications due to abdominal visceral injury and accelerates the recovery of digestive function. Early enteral nutrition may better meet the neonates’ needs for nutrition and minimize the risk of enterogenic infection (18).
This study demonstrated that timing of thoracoscopy, performed within 85 hours of birth for left-sided CDH repair, did not significantly affect the therapeutic outcomes of children with left-sided mild-to-moderate CDH.
There are limitations in the present study. First, considering the scattered distribution and the low incidence of CDH, besides the single-center design of the study, a sufficient number of cases with complete data could not be included for a short-term analysis. Second, due to the small sample size and incomplete data, it was difficult to reach an accurate conclusion. Third, this trial had a short follow-up period, and no long-term outcomes, such as postoperative recurrence rate, mortality, pneumonia, thoracic deformity, or scoliosis, could be observed. Finally, the appropriate surgical time may be much longer than 85 hours. Therefore, larger multicenter studies with longer follow-ups are required to validate the present findings.