1. Background
Periventricular/intraventricular hemorrhage (PVH/IVH), also known as germinal matrix/intraventricular hemorrhage (GMI/IVH), has a major impact on neurodevelopment due to its complications and sequelae (1, 2). It is the most common hemorrhage during the neonatal period, which can be localized periventricular or intraparenchymal (accompanied by ventricular dilatation or not) and rarely hydrocephalus. It can be detected using ultrasound (US) or magnetic resonance imaging (MRI) (3).
Recent studies have shown that transfontanellar neurosonography is a method of choice in the diagnosis and monitoring of PVH/IVH (4). With the more intense application of MR of endocranium, a number of papers comparing these 2 diagnostic methods have been published. MR has its advantages due to a more precise visualization of anatomical structures and pathological changes, as well as due to the stationary of the findings. Its disadvantage is due to the necessity of sedation. If the newborn is in an incubator, oxygen-dependent, it is not possible to perform an MRI examination, as opposed to neurosonographic examinations that can be performed repeatedly whenever needed. Computerized tomography should be avoided due to high doses of radiation (5).
The severity of PVH/IVH, 4 grades, is described by the Papile classification (6, 7) as follows:
- Grade I: Isolated germinal matrix hemorrhage and periventricular hemorrhage.
- Grade II: IVH without ventricular dilatation that denotes occupying less than 50% of the lateral ventricle volume.
- Grade III: IVH with ventricular dilatation that denotes occupying more than 50% of the lateral ventricle volume.
- Periventricular hemorrhagic infarction (PVHI) and intraventricular and intraparenchymal hemorrhage denote the propagation of hemorrhage in the periventricular white matter, formerly referred to as grade IV.
Grade I and grade II are considered mild, and grade III and PVHI are considered severe.
The transfontanelar neurosonography is most commonly used for diagnosing PVH/IVH, although the Papile classification was based on computed tomography (CT) images. In recent years, we can find many suggestions for replacing the Papile classification with other more precise and descriptive ones (8, 9); however, it is still widely used, and the decision for treatment depends on those findings (10).
Complications of PVH/IVH are ventriculomegaly, posthemorrhagic hydrocephalus (obstructive and non-structural), subdural effusion, ventricular septicity, destruction of a subependymal germinative matrix with the emergence of porencephalic cysts, brain atrophy, periventricular venous infarcts, and meningitis as the least frequent (11).
The PVH/IVH sequelae are a central disorder of coordination, later cerebral paralysis, dystonia, hemiparesis, hemiplegia, cognitive disorders, psychomotor retardation, neonatal convulsions, visual impairment, hearing impairment, behavioral disorders, fine motor skills disorder, speech disorder, and epilepsy. Recently, hyperkinetic syndrome, attention-deficit/hyperactivity disorder (ADHD), has become increasingly frequent. Sequelae occur due to lesions of the cerebral parenchyma and hydrocephalus (11).
The risk of posthemorrhagic hydrocephalus has increased, and 75% of the examined cases with parenchymal hemorrhage had significant neurodevelopmental disorders (12).
The prognosis depends on the degree of hemorrhage and other associated factors (gestation week, how the delivery ended, follow-up respiratory distress syndrome (RDS), convulsions, association with periventricular leucomalacia (PVL), and low Apgar score. Also, Regenerative potentials of the brain and early rehabilitation are important for the prognosis.
Infants with IVH associated with PVL have a higher risk of developing neurodevelopmental disorders. Preterm delivery and low gestation age are antenatal predictors for both entities (13). A rare but severe complication of PVL is cystic PVL, which often leads to brain atrophy.
2. Objectives
This study aimed to determine the significance of monitoring neurosonographic findings, the frequency of examination, and the length of the monitoring period in children diagnosed with PVH/IVH.
3. Methods
This clinical observational analytical, retrospective cohort study was performed on 102 children with neurosonographic diagnosis (PVH/IVH) during the neonatal period and the control group of 102 children with a normal neurosonographic finding. The examinations were conducted on the third, seventh, fourteenth, and twenty-first days after birth, as well as in the fourth and sixth weeks and third and sixth months after birth.
The study population consisted of patients referred to a pediatric clinic in Kragujevac. Collected data included a history of diseases, discharge lists, findings of neurosonographic examinations performed in the outpatient clinic, and other necessary medical documentation.
Demographic data included 18- to 41-year-old mothers with diseases such as gestational diabetes (12.7%), arterial hypertension (13.7%), anemia (9.8%), thrombophilia (6.9%), birth weight (900 - 4500 g), birth week [26 - 38 weeks; male (66.7%) and female (33.3%)], delivery type [vaginal delivery (60.8%), cesarean section (35.3%), and vacuum extraction (3.9%)].
Neurosonographic examinations were performed according to the guidelines of neurosonographic examinations defined by the American Institute of Ultrasound in Medicine (AIUM) (14). The sector probe, 3.5 and 5 MHz, was used for the examination. Open anterior fontanelle was used as a window for US scanning. Recordings were done on gray-scale imaging.
The kappa measure of agreement test was used to compare and determine the matching of the results. SPSS version 20 (SPSS Inc, Chicago, Ill, USA) was used for statistical analysis.
4. Results
In the group of patients, the percentage by the grades of PVH/IVH (according to the Papile classification) is as follows: Grade I, 54.9%; grade II, 21.6%; grade III, 14.7%; PVHI, 9.8%. Besides, PVH/IVH appears more often associated with PVL than alone in 69.6%, particularly grade I (44.6%) and grades II, III, and PVHI (100%).
Hemorrhages in grades I and II are more common (76.5%) and have fewer complications and sequelae than grade III and PVHI hemorrhages.
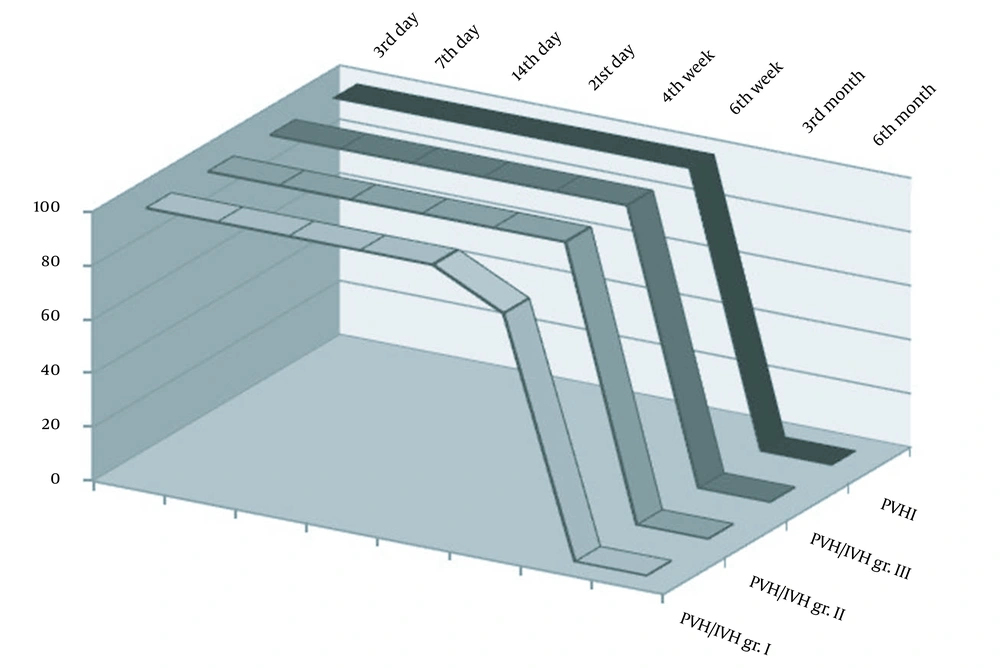
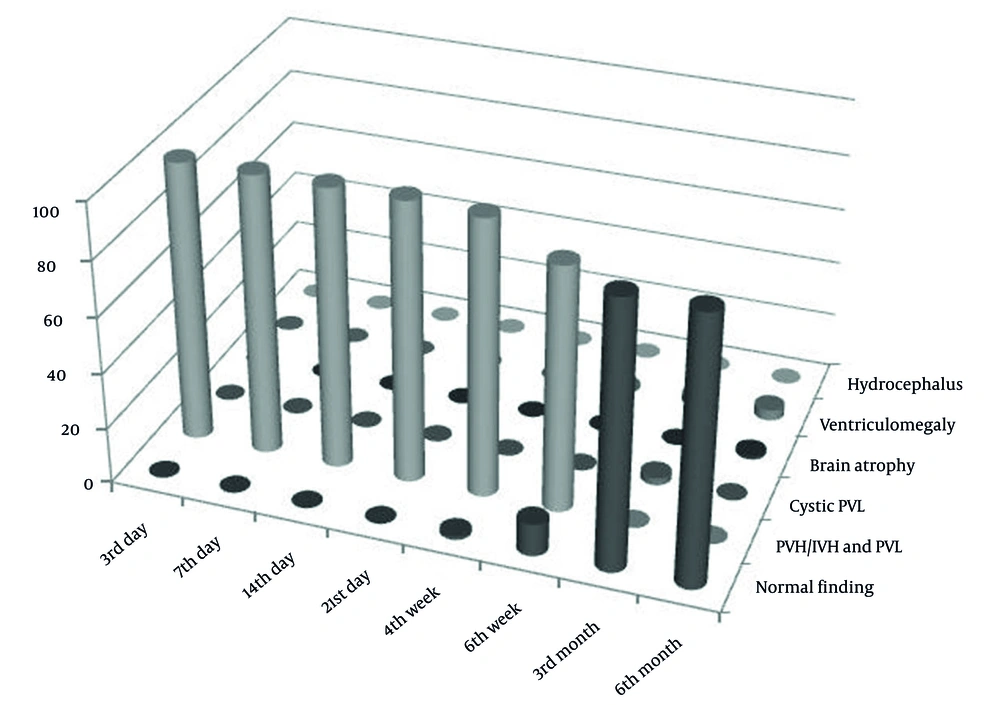
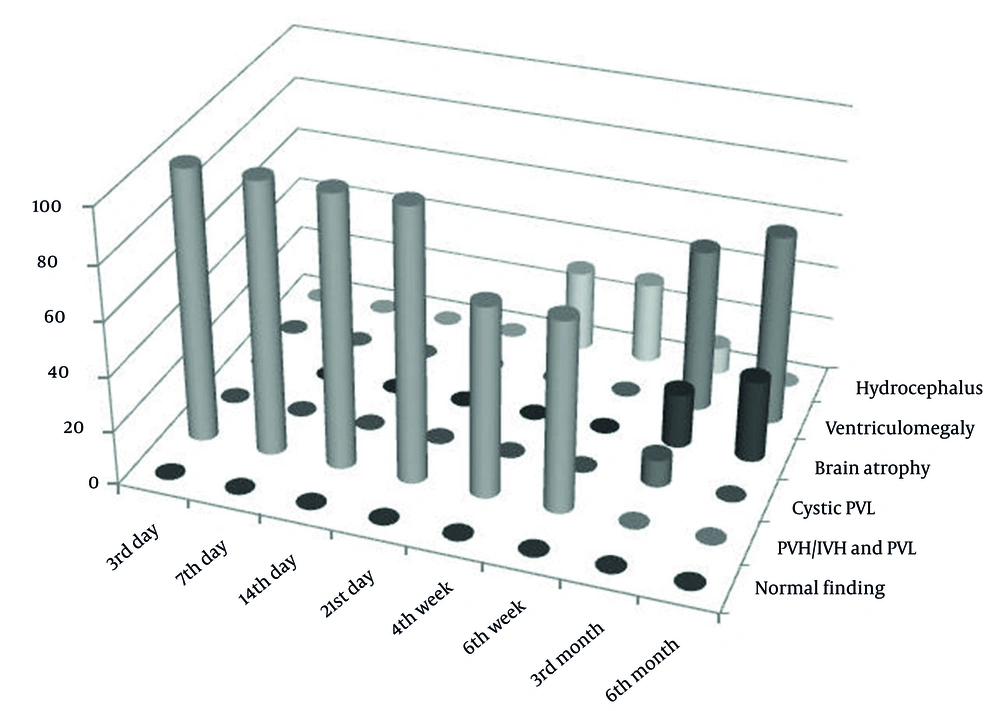
In the category of PVH/IVH grade I, after the third month, the normalization of neurosonographic findings was observed in all patients, while in PVH/IVH grade II, it occurred in 81.8% of cases. Contrary to that, in the category of PVH/IVH grade III, after the third month, the normalization of findings was observed in 14.3% of cases, while no normal findings were found in the PVHI category (Figure 1). In these categories, the pathological finding was longer maintained and transformed to ventriculomegaly, hydrocephalus, brain atrophy, or cystic leukomalacia if it was associated with PVL. The incidence of neurosonographic findings in each category over time is given in Figure 1. The evolution of the hemorrhage grade I and PVHI during the observation is shown in Figures 2 and 3.
The periodical results of the examinations diagnosed with PVH/IVH that were performed over time were subjected to the test of the kappa measure of agreement test, with a significance threshold of 0.05, to determine when the most significant changes take place in the patient’s condition, to determine the optimum frequency of the examination. A statistically significant matching of the results between all examinations performed at the observed moments (r < 0.0005).
However, it was found that the agreement up to the fourth week was excellent (the kappa agreement was greater than 0.85); in the sixth week, it was very good (the kappa agreement was 0.79), while the agreement for the sixth week and the third month was very small (the kappa agreement was less than 0.1). After that, no major changes occurred (the kappa agreement between the third and sixth months was 0.88; Table 1).
| Variable | 3rd and 7th Days | 7th and 14th Days | 14th and 21st Days | 21st Day and 4th Week | 4th and 6th Weeks | 6th Week and 3rd Month | 3rd and 6th Month |
|---|---|---|---|---|---|---|---|
| Kappa measure of agreement a | 1 | 1 | 1 | 0.890 | 0.793 | 0.065 | 0.884 |
Kappa Measure of Agreement for the Consecutive Examinations
5. Discussion
With the advancement of medicine, intensive care, and therapy, an increasing number of preterm infants survive. However, neurodevelopmental disorders are the most significant due to PVH/IVH and periventricular leukomalacia.
Studies have found data on the incidence of very low birth weight preterm infants (less than 1500 g), which in most centers was 20% - 30% (15-17). The incidence of IVH was 15% as follows: Grade I, 50%; grade II, 17%; grade III, 11%; PVHI, 22%. IVH was the most common during the first week of life in 78% (18), and it is similar in different studies.
There is evidence that PVH/IVH can develop even more intrauterine, although hemorrhage commonly occurs after birth. Most studies have shown that PVH/IVH occurs most frequently in the first week of life, 50% of bleeding occurs within the first 24 hours of birth, and 90% of all bleeding occurs within the first 72 hours after birth (19). While in some studies, it has been mentioned that 2/3 of hemorrhages occur within the first 6 hours after birth; even in some other studies, it has been shown that it occurs in the first 3 days of life; 20% - 40% of hemorrhages occur during the first week of life (20, 21). Almost 50% of hemorrhages occur in the first 6 to 8 hours after birth, which are the most evident on the third day of life (22)
The clinical manifestation of PVH/IVH is different: Most children are asymptomatic, especially for grade I and II hemorrhages, or show subtle signs that are easily overlooked. The other extreme is a sudden and significant worsening of the general condition associated with anemia, glucose variation, metabolic or respiratory acidosis, apnea, hypotonia, and coma. Severe neurological disorders are more common in grade III and IV hemorrhages. Progression can be rapid and can result in shock or a lethal outcome. Between the 2 stated extremes, different degrees of manifestation of neurological and systemic disorders may occur (11).
Lemons et al. suggest that PVH/IVH grades I and II have far better outcomes than grades III or IV (23). Grade III or IV intraparenchymal hemorrhages are associated with posthemorrhagic hydrocephalus (24). Grade IV hemorrhage has a high mortality rate up to 80%, permanent sequelae, motor deficit as high as 100%, and cognitive impairment of 85%.
Chinta showed that cranial sonography was abnormal in 51 (25.62%) cases of 199 asymptomatic premature infants (25). Therefore, it was suggested by Ballardini et al. that cranial ultrasonography should be considered universal screening for all preterm neonates and a uniform protocol for early detection of different cranial abnormalities (26). Harris et al. indicated that screening should be performed in preterm infants at 30 weeks or less, as the recommendation given by the American Academy of Neurology and the Child Neurology Society (27).
Intracranial hemorrhage is optimally diagnosed between the fourth and seventh days after birth using US, with follow-up until the 14th day of life. The ventricular dilation could be confirmed as early as on the 14th day, with a consequent follow-up for 3 months. To predict long-term sequelae, performing a routine cranial US is recommended around the second and sixth week of life. Early findings could show signs of hemorrhagic lesions, and delayed findings could detect ventriculomegaly and cystic lesions. Those examinations should be performed and completed until 3 months of age or later. The majority of the detected cysts are evident within 60 days of birth. Upon identifying brain injuries in infants, it is clinically indicated to repeat USs (22). Our results support those studies.
According to the guidelines by Wezel-Meijler, it is recommended that serial neurosonography should be performed on days 1, 3, 7, 14, 21, and 28 and then every other week in preterm infants with a gestational age below 28 weeks or 1000 g, and with a gestational age above 28 weeks, it should be limited to days 1, 3, 7, 14, and 28 at 6 weeks. More examinations should be performed in the case of clinical or neurosonographical suspected hemorrhage with unsure findings (28). PVH/IVH is a characteristic of premature infants, but with maturing of the germinal matrix, what is known is that it is over until 36 weeks. However, we also diagnosed hemorrhage in neonates who develop more than 36 weeks; thus, performing US in term neonates is reasonable. In the meta-analysis by Mukerji et al. it was shown that severe grades of PIVH might be significantly associated with long-term neurodevelopmental outcomes than mild PIVH (29). Performing serial neurosonographic findings enables timely detection of deterioration of findings, which would timely prevent the sequelae caused primarily by the development of posthemorrhagic hydrocephalus, which causes the most severe neurodevelopmental sequelae. The optimal time to perform a neurosonographic examination is important for clinical practice and affects the reduction of unnecessary repetition of neurosonographic examinations because all other findings after the third month will be stationary, according to our results.
5.1. Conclusions
The application of neurosonographic examinations is justified for the diagnosis of PVH/IVH, which is necessary to monitor the evolution of the findings. The frequency of the neurosonographic examinations and the length of follow-up period are estimated based on the obtained findings.
The first neurosonographic examination should be conducted in the first 7 days after birth - not later than 14 days - because the incidence of hemorrhage is higher by the third day, followed by a decrease until the seventh day. The control examination should be conducted at the age of 4 - 6 weeks. If the pathological finding is maintained (ventriculomegaly, hydrocephalus, and cystic leukomalacia), it should be repeated once a week, once in 2 weeks, or every month (if necessary), and after the specified period until the stationary finding is observed. The most significant differences, as compared to the previous findings, occur in the third month.
When PVH/IVH is associated with PVL, the complications and sequelae are aggravated, and the pathological finding is maintained longer.
Considering that PVH/IVH can pass completely asymptomatic (ie, it can occur within the first 7 days after birth) and that hemorrhage occurs in term newborns, it is necessary to do a neurosonographic examination in the form of routine screening in the maternity ward, for early diagnosis and further monitoring of the evolution of hemorrhage to prevent possible complications and sequelae. If the finding is pathological, there is a higher probability and occurrence of disorders of neurological development.
In addition to hemorrhage and asphyxia, it is easier to detect other pathological findings (congenital anomalies, ventriculomegaly, congenital cysts and cysts of other etiology, calcification, arteriovenous malformations, infections, tumors, etc).
Prenatal and postnatal preventive measures reduce risk factors for the development of PVH/IVH. Simultaneous neurosonographic examinations and monitoring of neurological development enable timely implementation of conservative or surgical treatment and rehabilitation, which would not only reduce the possibility of occurrence of complications and sequelae, but also alleviate or eliminate them.