1. Background
Henoch-Schönlein purpura (HSP) is considered as one of the most common primary vasculitis in Asian children, especially in China (1). HSP is an autoimmune disease that causes systemic inflammation. The pathological features are related to the deposition of immunoglobulin (IgA) deposition in small blood vessels, accompanied by adverse reactions to the skin, joints, gastrointestinal tract (GI) system, kidneys, and other body systems and organs (2). As a self-limited disease, the prognosis of HSP is generally good. However, complications can lead to a poor prognosis, with HSP nephritis (HSPN) being the most severe complication. This serious complication occurs in about 40% of pediatric patients and usually occurs within 4 - 6 weeks of the onset of a typical purpura rash (3). Furthermore, the severity of nephritis in these patients directly affects their long-term prognosis (4).
High-mobility group box-1 (HMGB1) is a member of the HMG family (including HMGB1, HMGB2, HMGB3, and HMGB4) with a molecular weight of 25 - 30 kDa (5). HMGB1 is a nuclear protein that binds to DNA and acts as a co-factor for gene transcription, participating in a variety of biological processes, including transcription, DNA repair, differentiation, and development (6). Under pathological conditions, it is released from dead or activated cells and acts as an inflammatory agent through a variety of cell surface receptors, including RAGE and toll-like receptors (TLRs). As a powerful pro-inflammatory cytokine, it plays an important role in the pathogenesis of various inflammatory and autoimmune diseases.
HMGB1 single-nucleotide polymorphisms (SNPs) are associated with many autoimmune and inflammatory diseases. Gene polymorphisms of HMGB1 were related to the incidence of colorectal cancer (7), the risks reduction of lung cancer (8), the susceptibility and outcomes of sepsis (9), and the individual susceptibility to CWP (10) in the Chinese population. There is no report on the association between HMGB1 polymorphisms and HSP in the Chinese population.
Recently, studies have shown that HMGB1 is a key factor related to the occurrence and development of a variety of renal diseases (11). Clinical investigations also found that HSP patients had significantly increased serum levels of HMGB1 (12) and had abundant expression in damaged skin endothelial cells (13). Therefore, the relationship between HMGB1 and HSPN is worthy of further research.
2. Objectives
This study investigated whether HMGB1 polymorphism is associated with HSP susceptibility and clinical heterogeneity in children.
3. Methods
3.1. Study Population
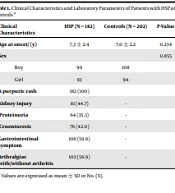
In this hospital-based case-control study, 182 patients with HSP were enrolled from the Children’s Hospital Zhejiang University School of Medicine between June 2011 and April 2014, in the eastern China. All patients with HSP were diagnosed according to the American College of Rheumatology criteria (14), and newly diagnosed HSP cases were included in this study and followed up for at least six months. Of these, 78 patients were diagnosed with HSPN, and 39 HSPN patients underwent renal biopsy. The standard of hematuria is more than five red blood cells per high-power field of vision in urine sediment. Proteinuria is defined as more than 150 milligrams of protein with over 24-hour urine collecting. Nephritis is defined as the presence of hematuria and/or proteinuria. Based on the criteria of the International Study of Kidney Diseases in Children, three patients were divided into grade I, 12 into grade II, 20 as grade III, 3 as grade IV, and 1 as grade V (15). Patients with any other autoimmune disorders or systemic inflammatory were excluded. Meanwhile, 186 healthy children (101 boys and 85 girls, with an average age of 7.0 ± 2.2 years) from the same hospital were selected as the control group. All subjects in this study were Han Chinese. Clinical characteristics and laboratory parameters were collected, including age and gender, neurological symptoms, infection history (before HSP), serum levels of albumin, creatinine, complement C3, C4, leukocyte, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and proteinuria. The Clinical characteristics and laboratory parameters of HSP patients and controls were provided in Table 1. All informed consents for genetic analysis were obtained from the enrolled investigators. This study was approved by the Ethical Committee of the Children’s Hospital of Zhejiang University School of Medicine (2017-IRB-041).
| Clinical Characteristics | HSP (N = 182) | Controls (N = 202) | P-Value |
|---|---|---|---|
| Age at oncet/ (y) | 7.3 ± 2.4 | 7.0 ± 2.2 | 0.234 |
| Sex | 0.855 | ||
| Boy | 99 | 108 | |
| Girl | 83 | 94 | |
| A purpuric rash | 182 (100) | ||
| Kidney injury | 81 (44.7) | - | |
| Proteinuria | 64 (35.3) | - | |
| Cruenturesis | 76 (42.0) | - | |
| Gastrointestinal symptom | 108 (59.6) | - | |
| Arthralgias with/without arthritis | 103 (56.9) | - |
a Values are expressed as mean ± SD or No. (%).
3.2. Single Nucleotide Polymorphisms Target of HMGB1 Gene Selection
Two tag single nucleotide polymorphisms (SNPs) were selected from the website of HapMap Project for HMGB1 (rs3742305 and rs9508752) for all minor allele frequencies (MAF) > 0.1 and an r2 threshold of 0.8 in the Han Chinese population.
3.3. Genomic DNA Extraction and Genotyping
DNA was extracted from white blood cells and centrifuged from EDTA anticoagulant blood samples. The DNA extraction kit was purchased from Tissuebank Biotechnology Co. Ltd, Shanghai, China, and operated according to the manufacturer's instructions. Samples were stored at -20°C until the end of polymerase chain reaction (PCR) analysis, and the extracts were stored at -80°C for a long time.
SNP genotyping was performed in a 384-well plate on the Sequenom MassARRAY platform (Sequenom, San Diego, USA), as described elsewhere (16). The primers for HMGB1 rs3742305 were 5′-ACGTTGGATGAAAAGAAGGCTGCGAAGCTG-3′ (forward) and 5′-ACGTTGGATGGTACAATCATACATCTGGCG-3′ (reverse). The primers for HMGB1 rs9508752 were 5′-ACGTTGGATGTGAATTGCCTGCAAGTGGTG-3′ (forward) and 5′-ACGTTGGATGATGCTAATCTTCCCCTAGTC-3′ (reverse). Genotype calling from real-time PCR was performed with MassARRAY RT software version 3.0.0.4 and MassARRAY Typer software version 3.4 (Sequenom).
3.4. Statistical Analyses
The SPSS software (version 25.0; IBM Inc. Armonk, NY, USA) was used for all statistical analyses. The allele, genotype, and haplotype frequencies were compared by the chi-square (χ2) test to determine statistical differences between cases and controls. The allele and genotype frequencies of patients with and without clinical characteristics of HSP were compared. Odds ratio (OR) and 95% confidence interval (CI) for each explanatory variable were calculated. The Hardy-Weinberg equilibrium was tested by χ2 goodness-fit test. Finally, Haploview 4.2 software was used to calculate the haplotype block and linkage disequilibrium (17).
4. Results
The genotype and allele frequencies of HMGB1 SNPs in HSP patients and controls are shown in Table 2. As shown, the distribution of the HMGB1 rs3742305 and rs9508752 genotypes obeyed the Hardy-Weinberg equilibrium in both patients and controls (P > 0.05). There were no significant differences in these two SNPs' genotype and allele frequencies between HSP patients and controls (P > 0.05). The two tag SNPs were located in the same haplotype block in haplotype analysis. Similarly, the haplotype of the HMGB1 gene had no significant correlation with HSP risk (P > 0.05).
| Polymorphisms | Control (N = 202) | HSP (N = 182) | OR (95% CI) | P-Value |
|---|---|---|---|---|
| rs3742305 | ||||
| Genotype | 0.98 | |||
| C/C | 136 (67.3) | 123 (67.6) | 1 | |
| C/G | 61 (30.2) | 55 (30.2) | 1.00 (0.64 - 1.55) | |
| G/G | 5 (2.5) | 4 (2.2) | 0.88 (0.23 - 3.37) | |
| Dominant model | 0.89 | |||
| A/A | 119 (58.9) | 106 (58.2) | 1 | |
| A/G-G/G | 83 (41.1) | 76 (41.8) | 1.03 (0.68 - 1.54) | |
| Recessive model | 0.86 | |||
| C/C-C/G | 197 (97.5) | 178 (97.8) | 1 | |
| G/G | 5 (2.5) | 4 (2.2) | 0.89 (0.23 - 3.35) | |
| Overdominant model | 1 | |||
| C/C-G/G | 141 (69.8) | 127 (69.8) | 1 | |
| C/G | 61 (30.2) | 55 (30.2) | 1.00 (0.65 - 1.55) | |
| rs9508752 | ||||
| Genotype | 0.96 | |||
| A/A | 119 (58.9) | 106 (58.2) | 1 | |
| A/G | 70 (34.6) | 63 (34.6) | 1.01 (0.66 - 1.55) | |
| G/G | 13 (6.4) | 13 (7.1) | 1.12 (0.50 - 2.53) | |
| Dominant model | 0.89 | |||
| A/A | 119 (58.9) | 106 (58.2) | 1 | |
| A/G-G/G | 83 (41.1) | 76 (41.8) | 1.03 (0.68 - 1.54) | |
| Recessive model | 0.78 | |||
| A/A-A/G | 189 (93.6) | 169 (92.9) | 1 | |
| G/G | 13 (6.4) | 13 (7.1) | 1.12 (0.50 - 2.48) | |
| Overdominant model | 0.99 | |||
| A/A-G/G | 132 (65.3) | 119 (65.4) | 1 | |
| A/G | 70 (34.6) | 63 (34.6) | 1.00 (0.66 - 1.52) | |
| Haplotype (rs3742305, rs9508752) | ||||
| CA | 266 (69.4) | 272 (70.9) | 1 | |
| CG | 50 (13.0) | 45 (11.8) | 0.89 (0.57 - 1.39) | 0.62 |
| GG | 41 (10.7) | 48 (12.6) | 1.12 (0.71 - 1.76) | 0.62 |
| GA | 26 (6.8) | 18 (4.7) | 0.68 (0.34 - 1.34) | 0.26 |
Abbreviations: OR, odds ratio; CI, confidence interval.
a Values are expressed as No. (%).
In addition, we assessed the genotype frequency of HSP patients, with and without clinical manifestations, in Table 3. There was no statistically significant difference in genotype frequency between the two types of HSP patients with or without typical clinical symptoms, such as renal manifestations, gastrointestinal involvement, and joint pain (P > 0.05).
| Polymorphisms | HSP with Kidney Injury | OR (95% CI) | P-value | HSP with Gastrointestinal Symptom | OR (95% CI) | P-Value | HSP with Arthralgia | OR (95% CI) | P-Value | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No (N = 81) | Yes (N = 100) | No (N = 108) | Yes (N = 73) | No (N = 103) | Yes (N = 78) | |||||||
| rs3742305 | ||||||||||||
| Genotype | 0.59 | 0.17 | 0.59 | |||||||||
| C/C | 57 (70.4) | 65 (65) | 1 | 70 (64.8) | 52 (71.2) | 1 | 71 (68.9) | 51 (65.4) | 1 | |||
| C/G | 23 (28.4) | 32 (32) | 1.22 (0.64 - 2.32) | 37 (34.3) | 18 (24.7) | 0.65 (0.34 - 1.28) | 29 (28.2) | 26 (33.3) | 1.25 (0.66 - 2.37) | |||
| G/G | 1 (1.2) | 3 (3) | 2.63 (0.27 - 26.00) | 1 (0.9) | 3 (4.1) | 4.04 (0.41 - 39.94) | 3 (2.9) | 1 (1.3) | 0.46 (0.05 - 4.59) | |||
| Dominant model | 0.44 | 0.36 | 0.61 | |||||||||
| A/A | 57 (70.4) | 65 (65) | 1 | 70 (64.8) | 52 (71.2) | 1 | 71 (68.9) | 51 (65.4) | 1 | |||
| A/G-G/G | 24 (29.6) | 35 (35) | 1.28 (0.68 - 2.40) | 38 (35.2) | 21 (28.8) | 0.74 (0.39 - 1.41) | 32 (31.1) | 27 (34.6) | 1.17 (0.63 - 2.20) | |||
| Recessive model | 0.41 | 0.15 | 0.45 | |||||||||
| C/C-C/G | 80 (98.8) | 97 (97) | 1 | 107 (99.1) | 70 (95.9) | 1 | 100 (97.1) | 77 (98.7) | 1 | |||
| G/G | 1 (1.2) | 3 (3) | 2.47 (0.25 - 24.25) | 1 (0.9) | 3 (4.1) | 4.59 (0.47 - 44.98) | 3 (2.9) | 1 (1.3) | 0.43 (0.04 - 4.24) | |||
| Overdominant model | 0.6 | 0.17 | 0.45 | |||||||||
| C/C-G/G | 58 (71.6) | 68 (68) | 1 | 71 (65.7) | 55 (75.3) | 1 | 74 (71.8) | 52 (66.7) | 1 | |||
| C/G | 23 (28.4) | 32 (32) | 1.19 (0.63 - 2.25) | 37 (34.3) | 18 (24.7) | 0.63 (0.32 - 1.22) | 29 (28.2) | 26 (33.3) | 1.28 (0.67 - 2.41) | |||
| rs9508752 | ||||||||||||
| Genotype | 0.88 | 0.36 | 0.59 | |||||||||
| A/A | 48 (59.3) | 57 (57) | 1 | 59 (54.6) | 46 (63) | 1 | 60 (58.2) | 45 (57.7) | 1 | |||
| A/G | 28 (34.6) | 35 (35) | 1.05 (0.56 - 1.97) | 42 (38.9) | 21 (28.8) | 0.64 (0.33 - 1.23) | 34 (33) | 29 (37.2) | 1.14 (0.61 - 2.13) | |||
| G/G | 5 (6.2) | 8 (8) | 1.35 (0.41 - 4.39) | 7 (6.5) | 6 (8.2) | 1.10 (0.35 - 3.49) | 9 (8.7) | 4 (5.1) | 0.59 (0.17 - 2.05) | |||
| Dominant model | 0.76 | 0.26 | 0.94 | |||||||||
| A/A | 48 (59.3) | 57 (57) | 1 | 59 (54.6) | 46 (63) | 1 | 60 (58.2) | 45 (57.7) | 1 | |||
| A/G-G/G | 33 (40.7) | 43 (43) | 1.10 (0.61 - 1.99) | 49 (45.4) | 27 (37) | 0.71 (0.38 - 1.30) | 43 (41.8) | 33 (42.3) | 1.02 (0.56 - 1.86) | |||
| Recessive model | 0.63 | 0.66 | 0.34 | |||||||||
| A/A-A/G | 76 (93.8) | 92 (92) | 1 | 101 (93.5) | 67 (91.8) | 1 | 94 (91.3) | 74 (94.9) | 1 | |||
| G/G | 5 (6.2) | 8 (8) | 1.32 (0.42 - 4.21) | 7 (6.5) | 6 (8.2) | 1.29 (0.42 - 4.01) | 9 (8.7) | 4 (5.1) | 0.56 (0.17 - 1.91) | |||
| Overdominant model | 0.95 | 0.16 | 0.56 | |||||||||
| A/A-G/G | 53 (65.4) | 65 (65) | 1 | 66 (61.1) | 52 (71.2) | 1 | 69 (67) | 49 (62.8) | 1 | |||
| A/G | 28 (34.6) | 35 (35) | 1.02 (0.55 - 1.89) | 42 (38.9) | 21 (28.8) | 0.63 (0.34 - 1.20) | 34 (33) | 29 (37.2) | 1.20 (0.65 - 2.22) | |||
Abbreviations: OR, odds ratio; CI, confdence interval.
a Values are expressed as No. (%).
5. Discussion
This study analyzed the distribution of HMGB1 labeled SNPs genotypes in HSP and control groups of Chinese children. It is suggested that the HMGB1 polymorphism and haplotype involved in this study were unrelated to HSP and its clinical manifestations.
As a pro-inflammatory factor, once it is released from necrosis or activated cells, HMGB1 can play a role through a variety of cell surface receptors. It not only has the pro-inflammatory effect itself but also can stimulate a variety of pro-inflammatory factors. In turn, pro-inflammatory factors can promote the secretion of HMGB1, and a positive feedback loop is formed. In the later stage of the inflammatory response, this positive feedback plays an important role in the maintenance of inflammatory response (18). Moreover, HMGB1, as a powerful heparin-binding protein, can affect the coagulation function of the body and promote disseminated intravascular coagulation (DIC) (19). At the same time, HMGB1 can also affect the normal function of vascular endothelial cells (20).
Gene polymorphisms of HMGB1 were associated with pneumonia, sepsis, rheumatoid arthritis, and some cancers (8, 9, 21, 22). However, this study's results showed no significant association between the major clinical manifestations (such as kidney injury) and the HMGB1 genotype in the Chinese population. Therefore, considering that the HMGB1 gene is not related to HSP, we believe that the possibility of HMGB1 gene variation participating in the pathogenesis of HSP is low. The increased expression of HMGB1 is only a pathological change related to HSP disease.
HSP is a heterogeneous disease with complex and multiple clinical manifestations. Unfortunately, the exact pathogenesis and etiology of HSP remain a mystery. Studies suggest that HSP may be caused by a variety of factors, including infectious and genetic factors (23). It is believed to be an immunoglobulin A (IgA) - related immune complex-mediated leukocytic fragmentative vasculitis characterized by perivascular leukocyte infiltration, often accompanied by purpura rash, arthritis, renal involvement, and abdominal pain. Increased production of numerous cytokines and chemokines has been found to be present in the circulation of HSP patients (24). At the same time, some studies have observed in clinical practice that the expression of HMGB1 is increased in the serum of HSP patients and in the endothelial cells of the lesion site (12, 13).
HSP-related SNPs research has always been one of the hotspots. It suggested that MCP1/CCL2 2518 TT genotype and T allele were associated with HSP risk (25). The HSP70-2 and TNF-α gene polymorphisms were associated with HSP in children (26). There is an association between HLA DRB1 and HLA DRB1 gene polymorphisms and susceptibility to HSP (27). The iNOS polymorphisms are associated with the risk of HSP and may strongly contribute to the genetic basis of individual differences in the progression to nephritis among children with HSP in the Chinese Han population (28). In the MEFV gene, the variants in rs3743930 and interaction between rs3743930 and rs28940580 were associated with increased HSP risk in Chinese children (29). Our team also confirmed that the rs2275913 polymorphism of the IL17A gene was associated with susceptibility to HSP in Chinese children (30). On the other hand, some gene polymorphisms may hardly play a role in susceptibility to HSP, such as codon 54 polymorphism in the MBL gene supported by DURMAZ (31) and SNPs of IL-8 (32), IL-10 (33), and TLR4 (34) reported by our team.
This study mainly discussed the relationship between gene polymorphisms of HMGB1 and HSP and the clinical manifestations in the Chinese population, which is the first time in China. In order to obtain as much information as possible about the genetic variation of the HMGB1 locus and to exclude the possibility of missing other potential functional polymorphisms, we selected two marker SNPs from the HapMap database. However, our research had some drawbacks. First, the sample size was relatively small, which may lack sufficient statistical capacity to reflect the true correlation between the two. Second, the study population only included the Han Chinese population, and the results are not generalizable to other ethnic groups.
To sum up, this study suggests that genetic variation of the HMGB1 gene is unlikely to confer susceptibility to HSP, based on the result that polymorphism of HMGB1 gene is not correlated with HSP and clinical manifestations in Chinese children. However, due to the homogenous ethnic background of the subjects and the small sample size in this study, the results should be interpreted with caution. Therefore, further genetic studies are needed in different populations with large sample sizes to further explore the relationship between HMGB1 and HSP gene variations.