1. Background
Celiac disease (CD) is a complex autoimmune disorder in which patients suffer from a wide variety of intestinal and extra-intestinal problems. Symptoms usually present after gluten consumption, the major protein in wheat, rye, and barley, in genetically predisposed individuals. Complexes of gluten peptides and tissue transglutaminase trigger an autoimmune reaction that results in variable degrees of small bowel inflammation (1, 2).
Although it was supposed that CD's manifestations are limited to the gastrointestinal tract at the time of recognition, these days, more and more non-classic extra-intestinal symptoms are being introduced, especially in the pediatric settings. Short stature, fatigue, and headache are among the most common extra-intestinal symptoms in children. It should be mentioned that nowadays, due to advancements in diagnostic techniques, more subclinical asymptomatic cases are being detected (3, 4). Moreover, studies have revealed that attributing to a higher risk of cardiovascular diseases and malignancies, mortality risk is greater in CD patients (5).
CD is a common disease and mainly presents from early childhood; however, it may be undiagnosed, particularly in subclinical cases. A recent meta-analysis revealed a significantly higher prevalence of the disease in children compared to adults (0.9% vs. 0.5%) (6, 7).
It has been proposed that CD manifestations cannot be controlled except by following a life-long strict gluten-free diet (GFD), which usually reduces clinical symptoms and morbidity and increases nutritional parameters, including body weight and bone density. Children on a strict GFD show faster and higher rates of symptoms' resolution compared with adults. However, previous studies have shown persistent symptoms and histologic signs of intestinal damage, as well as low patient satisfaction, high costs, and non-adherence, which make the therapy imperfect (4, 8, 9).
2. Objectives
Considering the remarkable prevalence of CD and its multisystem effect in children, it is essential to assess the mere route of its treatment. In this study, we aimed to investigate GFD non-adherence and its most important causes among children and adolescents in Shiraz, Iran.
3. Methods
3.1. Population
This cross-sectional study was conducted on children aged 2.5 - 14 years old with a confirmed diagnosis of CD and on a GFD for more than 12 months. A total of 187 children diagnosed CD referred by a gastroenterologist to Shiraz Celiac Clinic were included in the study. The children were enrolled by census between 2018 and 2019 and assessed for dietary compliance followed by an interview.
Exclusion criteria were unwillingness to participate, IgA level less than 0.006 g/dL known as Ig A deficiency, and incomplete records.
As standard clinical care, all patients had received counseling about a GFD from an expert general practitioner. A retrospective chart review of these children was performed to collect the anthropometric data and the serologic tests. Dietary compliant and non-compliant groups were compared and assessed for factors affecting dietary compliance.
All the included participants were interviewed using a predefined data collection tool. Demographic characteristics (eg, age, sex, etc.), family-related data (eg, number of siblings, the educational and economic status of parents, etc.), and medical history (eg, age at the time of diagnosis, symptoms, coexisting medical diseases, level of IgA anti-transglutaminase antibodies (anti-tTG), pathologic examinations, etc.) were the main parts of the interview. Patients were also asked about adherence to GFD during last week, according to which they were classified into two strong and poor adherent groups; the major causes of non-adherence were also collected. Moreover, the examiner measured anthropometric features (weight (kg), height (cm)) at the time of the interview. Anthropometric data and the level of anti-tTG at the time of diagnosis were also collected from medical records.
All patients were diagnosed based on the estimation of anti-tTG using the Aeskulisa kit (Germany), along with the ELISA method. A titer of 18 IU/mL or higher was considered positive anti-tTG and Marsh type 2 or more severe in histological evaluation (10-12). The histological findings were classified according to the Oberhuber-modified Marsh classification (13).
3.2. Ethical Approval
This study was conducted according to the Helsinki declaration. Written informed consent was obtained from the parents of all children, and the data were kept confidential. The ethical committee of Shiraz University of Medical Sciences approved the survey (IR.SUMS.REC.1398.730).
3.3. Statistical Analyses
Continuous and categorical variables were presented as mean ± SD and number (percentage), respectively. Data were analyzed via descriptive statistics, independent sample t-test, and chi-square test by SPSS for Windows (Version 16.0. Chicago, SPSS Inc). Predictability of the factors was assessed using binary logistic regression analysis with the entering method to determine the best predictors of compliance. P-values less than 0.05 were regarded as statistically significant.
4. Results
In this study, 187 children in the age range of 2.5 to 14 years with the mean age of 10.9 ± 2.7 years (70 (37.4%) males and 116 (62%) females) were included. Among the patients, 42.2% (n = 79) did not adhere to GFD.
The demographic and clinical characteristics of children based on the adherence or non-adherence to GFD are listed in Table 1. Mean age and mean age at diagnosis time were 9.96 ± 2.79 and 7.91 ± 5.73 in the adherent group and 10.23 ± 2.64 and 7.12 ± 3.01 in non-adherent group, respectively. Based on these results, there was no statistically significant difference in terms of age (P = 0.503) and age at diagnosis time (P = 0.263) and follow-up time (0.082) between the two groups.
| Variables | Adherence Group (N = 108) | Non-adherence Group (N = 79) | P-Value |
|---|---|---|---|
| Current age in years | 9.96 ± 2.79 | 10.23 ± 2.64 | 0.503 |
| Age at diagnosis time in years | 7.91 ± 5.73 | 7.12 ± 3.01 | 0.263 |
| Follow-up time | 2.57 ± 1.93 | 3.11 ± 2.19 | 0.082 |
| Gender (male) | 71 (66.4) | 45 (57.0) | 0.191 |
| Number of siblings | 1 (1-2) | 1 (1-2) | 0.987 |
| Living condition | 0.921 | ||
| With parents | 104 (97.2) | 76 (97.4) | |
| With father | 3 (2.8) | 2 (2.6) | |
| Father education | 0.187 | ||
| Under diploma | 76 (71.0) | 46 (59.0) | |
| Diploma | 19 (17.8) | 17 (21.8) | |
| College | 12 (11.2) | 15 (19.2) | |
| Mother education | 0.091 | ||
| Under diploma | 74 (69.2) | 49 (62.8) | |
| Diploma | 24 (22.4) | 14 (17.9) | |
| College | 9 (8.4) | 15 (19.2) | |
| Residency | 0.957 | ||
| Urban | 90 (84.9) | 66 (84.6) | |
| Rural | 16 (15.1) | 12 (15.4) | |
| Housing situation | 0.419 | ||
| Proprietary | 71 (67.6) | 57 (72.2) | |
| Leased | 26 (27.6) | 16 (20.3) | |
| Others | 5 (4.8) | 6 (7.6) | |
| Monthly household Income (in Rials per million | 0.642 | ||
| ≤ 15 | 22 (21.2) | 19 (24.1) | |
| > 15 | 82 (78.8) | 60 (75.9) | |
| Current symptoms | 0.002 | ||
| Yes | 20 (18.5) | 31 (39.2) | |
| No | 88 (81.5) | 48 (60.8) | |
| Years since having symptom to diagnosis | 0.171 | ||
| < 1 year | 96 (90.6) | 71 (91.0) | |
| 1 - 3 | 4 (3.8) | 6 (7.7) | |
| > 3 | 6 (5.7) | 1 (1.3) | |
| Years since diagnosis | 0.529 | ||
| 1 - 3 | 70 (67.3) | 49 (62.8) | |
| > 3 | 34 (32.7) | 29 (37.2) | |
| Comorbidities | 0.987 | ||
| Chronic diarrhea | 11 (33.3) | 6 (33.3) | |
| Diabetic type 1 | 16 (48.5) | 8 (44.4) | |
| Thyroid disease | 3 (9.1) | 2 (11.1) | |
| Other | 3 (9.1) | 2 (11.1) | |
| Family member with celiac disease | 0.015 | ||
| Yes | 18 (16.7) | 4 (5.1) | |
| No | 90 (83.3) | 75 (94.4) | |
| Receiving diet recommendation | 0.511 | ||
| Yes | 90 (95.7) | 64 (95.5) | |
| No | 4 (4.3) | 3 (4.5) | |
| Pathology result | 0.538 | ||
| 1 | 2 (1.9) | 4 (5.1) | |
| 3A | 35 (32.4) | 26 (32.9) | |
| 3B | 47 (43.5) | 29 (36.7) | |
| 3C | 24 (22.2) | 20 (25.3) |
a Quantitative data were presented as mean ± SD for normal data and median (IQR) for non-normal data. Qualitative data were presented as number (%).
Among the patients, 66.4% of adherent and 57% of non-adherent group were male, with no gender difference between the two groups (P = 0.191). Also, the two groups did not have significant differences regarding the number of siblings (P = 0.987), living condition (P = 0921), father’s education (P = 0.187), mother’s education (P = 0.091), residency (P = 0.957), housing situation (P = 0.419), and monthly income (P = 0.642).
Significantly more symptoms were reported in children who did not adhere to GFD properly (39.2% vs. 18.5%, P = 0.002). On the other hand, years since having symptom to diagnosis (P = 0.171), years since diagnosis (P = 0.529), comorbidities (P = 0.987), and receiving diet recommendations (P = 0.511) had no significant differences between the two groups.
A higher family history of CD was seen among the adherent group compared to the non-adherent group (18 (16.7%) vs. 4 (5.1%); P = 0.015). There was no significant difference between the two groups according to pathology results (P = 0.538) (Table 1).
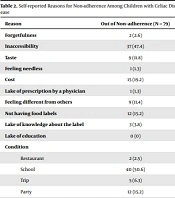
While the parents of most of the non-adherent children (47.4%) stated that the reason for non-adherence was improper access, some other parents stated high costs and lack of proper food labels. Most (50.6%, n = 40) non-adherent children did not adhere to GFD at school (Table 2).
| Reason | Out of Non–adherence (N = 79) |
|---|---|
| Forgetfulness | 2 (2.6) |
| Inaccessibility | 37 (47.4) |
| Taste | 9 (11.8) |
| Feeling needless | 1 (1.3) |
| Cost | 15 (19.2) |
| Lake of prescription by a physician | 1 (1.3) |
| Feeling different from others | 9 (11.4) |
| Not having food labels | 12 (15.2) |
| Lake of knowledge about the label | 3 (3.8) |
| Lake of education | 0 (0) |
| Condition | |
| Restaurant | 2 (2.5) |
| School | 40 (50.6) |
| Trip | 5 (6.3) |
| Party | 12 (15.2) |
| Camp | 1 (1.3) |
| Others | 19 (24.0) |
The results of anthropometric data are given in Table 3. As seen in this table, the mean weight at the time of diagnosis (P = 0.008) and the current weight (P = 0.023) in non-adherence group was much lower than adherent group. However, there was no significant difference between the two groups regarding change of weight (P = 0.141). According to height at the time of diagnosis and current age, there was no significant difference between the two groups (P > 0.05). Meanwhile, body mass index (BMI) was higher in adherent children at diagnosis (P = 0.006) and current time (P = 0.022) (Table 3).
| Variables | Groups | P-Value | |
|---|---|---|---|
| Adherence (N = 108) | Non–adherence (N = 79) | ||
| Weight at diagnosis (kg) | 27.70 ± 11.97 | 23.60 ± 7.50 | 0.008 |
| Current weight (kg) | 31.77 ± 12.39 | 28.19 ± 8.33 | 0.023 |
| Change | 4.09 ± 3.97 | 4.95 ± 3.55 | 0.141 |
| Height at diagnosis (cm) | 128.26 ± 18.92 | 124.43 ± 17.05 | 0.160 |
| Current height (cm) | 134.44 ± 18.61 | 132.14 ± 16.41 | 0.390 |
| Change | 5.85 ± 6.76 | 7.65 ± 5.66 | 0.061 |
| BMI at diagnosis (kg/m2) | 16.06 ± 3.38 | 14.87 ± 1.98 | 0.006 |
| Current BMI (kg/m2) | 16.96 ± 3.48 | 15.91 ± 2.33 | 0.022 |
| Change | 0.89 ± 1.81 | 1.04 ± 1.61 | 0.582 |
We collected the anti-tTG from the medical records of the children. The results showed no significant differences between the two groups before and after starting GFD (P = 0.052 and P = 0.433, respectively). There was a significant difference in changes between the two groups (P = 0.037), and a significant decrease within each group (P < 0.001) (Table 4).
| Variables | Groups | P-Value | |
|---|---|---|---|
| Adherence (N = 99) | Non–adherence (N = 71) | ||
| Log (IgAtTG) | |||
| Before | 4.78 ± 1.09 | 5.07 ± 0. 89 | 0.052 |
| Current | 2.30 ± 1.23 | 2.14 ± 1.53 | 0.433 |
| Change | -2.47 ± 1.41 | -2.93 ± 1.51 | 0.037 |
| P-value | < 0.001 | < 0.001 | - |
a We used the logarithm of IgAtTG due to its non-normality.
5. Discussion
The only effective method to control CD is strict adherence to a GFD. It is usually measured by checking symptoms, determining serum anti-tTG autoantibodies, or by interview; however, none of the techniques are quite reliable (14).
Based on the World Health Organization (WHO) definition, the associated factors of medication adherence in chronic disease are related to the disease, the patient, the treatment, the health system or health team, and the socioeconomic characteristics (15). In this study, we aimed to survey the prevalence and associated factors of GFD adherence in children with CD to estimate and control the disease in a more efficient way.
In our study, less than half of children with CD were non-adherent to a GFD, which is consistent with some previous studies (14, 16, 17). However, some studies demonstrated lower rates of non-adherence (18, 19). The rates vary based on the detecting methods, eg, adherence to GFD was reported 44% and 30.1% based on blood autoantibodies (anti-tTG and endomysial antibodies) and the adherence questionnaire, respectively in the study by Mehta et al. (14).
Based on anti-tTG level and histopathologic examinations, there was no significant difference between the two groups before and after treatment with a GFD for one year. However, there was a significant difference in changes between the two groups, and there was a significant decrease within each group. Recent research reported that a high level of anti-tTG was seen only in 43% of children with persistent enteropathy on biopsy, and on the other hand, a negative result of anti-tTG did not mean proper adherence or mucosal recovery (14, 20). Moreover, in longitudinal studies, mucosal recovery was associated with tight adherence to GFD, and our insignificant relation may be due to the nature of cross-sectional studies and their limitations (21, 22).
In this study, the demographic, economic condition, and clinical features of the two groups were not statistically different except having a family member with CD and current symptoms. Mehta et al. showed a similar pattern, in which there was no significant difference between adherent and non-adherent groups in terms of demographic, clinical, and laboratory characteristics (14). According to another study, age at presentation, nuclear families, mother's education, and a better knowledge of CD among the parents significantly affected compliance (23). Mager et al. mentioned that age, ethnicity, and gastrointestinal symptoms were also associated with adherence to the GFD in children with CD (24).
There is no consensus among the studies regarding the effects of age and gender and their impact on following a GFD. In our study, the majority of cases were male, and they had better compliance to GFD, which is inconsistent with a study by Charalampopoulos et al. (25) and congruous with the study by Rodrigues et al. (18). These two studies reported a higher non-adherence to GFD among adolescents, which is compatible with this study, though this factor was not statistically significant (25, 18).
Having symptoms results in higher adherence; in other words, patients with higher adherence experience lower symptoms. The experience-based result of symptoms was not found in our study, which may be due to the cross-sectional nature of the study. Since cross-sectional studies offer a snapshot of a single moment in time, they cannot identify a cause-and-effect relationship.
Most of the participants were suffering from a symptom for less than 12 months, and the period had no significant difference between adherent and non-adherent groups, that is less than the time span reported in another study (24 months) (18); however, the time was almost similar with two other studies (25, 26).
The most common reasons for non-adherence to the GFD were inaccessibility, lack of food labels, and high cost. These reasons were similar to some previous studies, which show the inattention of authorities (16, 27). In comparison with another study in children and adolescents with chronic liver disease in Shiraz, Iran, forgetfulness was known as the most common reason for non-adherence to the medications. We can attribute this issue to different medications, different methods of following a diet, and the different nature of the diseases (28).
However, in a study, no association was found between environment (eg, friends' house and birthday parties, home, and school) and adherence (18). Another study reported higher noncompliance during travel, at school, and family and marriage parties (23). To overcome this problem, it has been recommended to educate the parents to prepare gluten-free food for school and other social events to avoid meals containing gluten.
Several different ideas are on the weight change of children with CD on the GFD. This study revealed a significant difference between the two groups regarding the mean weight at the time of diagnosis and the current weight, which was much lower in the non-adherent group. Rodrigues et al. reported the threat of excessive weight gain among children with CD, mainly two years after starting GFD, which may have several consequences (18). In a retrospective study, while weight change improved in the children in the US, minor increases in overweight and notably underweight children with CD was reported in Italy. The accessibility of GFD and cultural variation in food preparation could be a reason for different results (29). Furthermore, the adherent group had a significantly higher BMI at both diagnosis time and the time of the study; however, the change in BMI was not significant. This is consistent with a previous study from this center and a recent meta-analysis (8, 30).
To the best of our knowledge, this study is the most extensive study evaluating adherence and associated factors among children with CD in Iran. However, like any cross-sectional study, our data are prone to recall bias.