1. Background
Type 1 diabetes mellitus (T1DM), the predominant type of diabetes in children, results from the autoimmune destruction of the β cells of the endocrine pancreas (1). Diabetes is one of the fast-growing global health emergencies as in 2021, over 1.2 million children and adolescents had T1DM (2). Despite the recent efforts to restore the activity of pancreatic β cells, insulin injection is still common and recommended universal treatment for this disease (3). However, repeated daily injections, weight gain, and hypoglycemia are the most common problems of insulin therapy in patients with T1DM (4, 5).
In recent decades, the development of insulin analogs by restructuring has led to modified pharmacodynamics and pharmacokinetics properties to overcome some of the disadvantages of conventional insulins (6, 7). These new analogs have been commonly applied in recent years and have some advantages over human insulins, especially the lower risk of hypoglycemia (7-10). Among the insulin analogs, detemir is long-acting, FDA-approved insulin for 2 - 6 years old children, which is used to maintain basal insulin levels. The effect of detemir may be maintained for up to 20 hours, but this duration is somehow determined by the injected dose (6, 11).
Some studies compared the efficacy of neutral protamine hagedorn (NPH) insulin with detemir, glargine with detemir, and even twice-daily detemir injections with once-daily in children or adults (12-17). Studies comparing once or twice-daily insulin detemir injections have mostly been in the adult age group. The results of these studies showed no difference in efficacy and HbA1c. Therefore, it is reasonable to recommend a once-daily regimen (13, 14). A study comparing the efficacy and side effects of the two methods in children age group by Nimri et al. stated that twice-daily detemir had no clinical advantage over once-daily. It should be mentioned that the latter research was not a randomized study (15).
2. Objectives
Briefly, although twice-daily injections are more prevalent than once-daily in pediatric diabetic patients, the lack of strong evidence for comparing the efficiency or side effects of the two methods is a concern. Therefore, the purpose of this study was to compare the effectiveness of once-daily and twice-daily insulin detemir injections in young children with T1DM.
3. Methods
3.1. Study Design
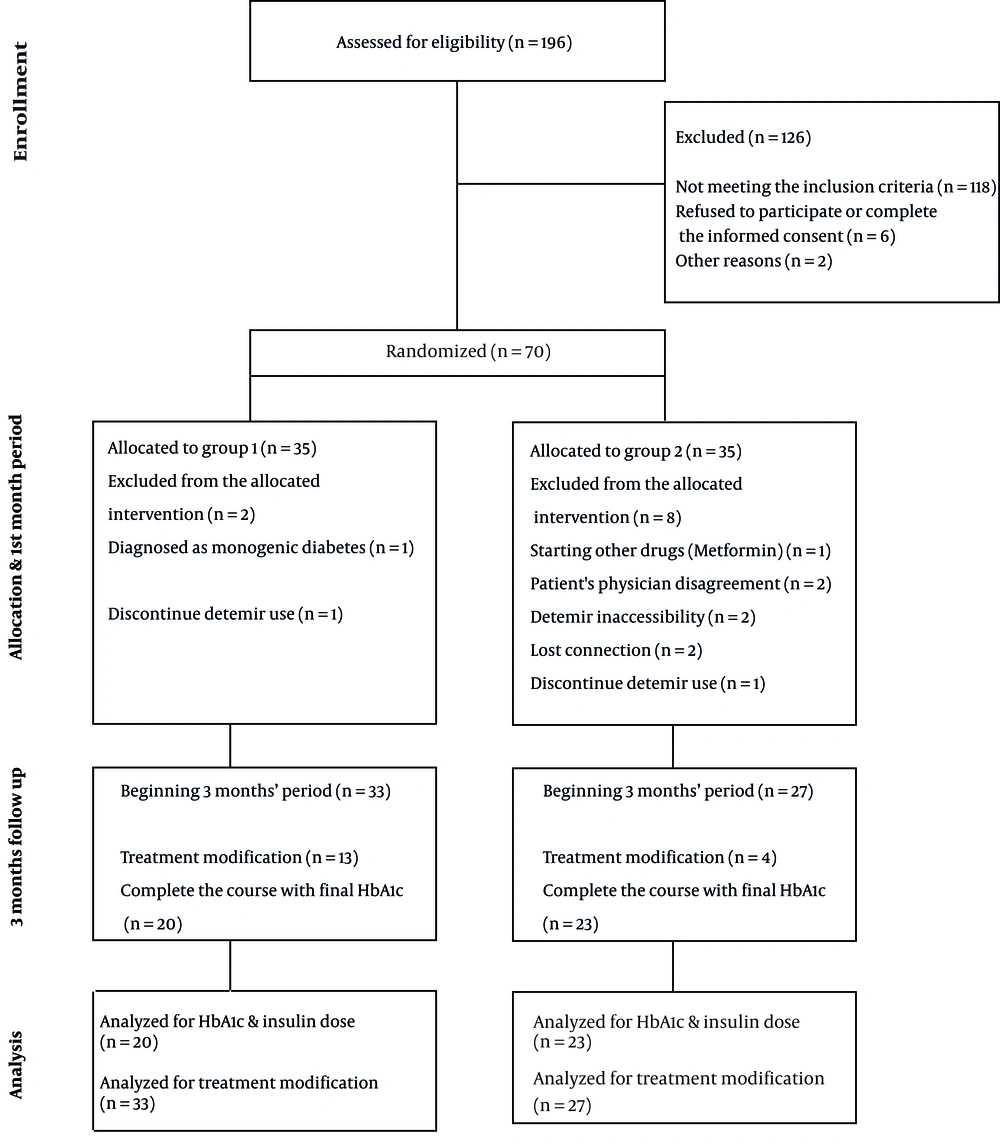
This randomized clinical trial was performed on children with T1DM diagnosed before the age of 6 years. In this study, informed consent was obtained from the parents of patients who met the inclusion criteria and referred to the Children's Medical Center. The participants were randomly assigned into two groups once-daily (group 1) and twice-daily (group 2) detemir insulin injection using block randomization. The used insulin was detemir (Levemir®, Novo Nordisk A/S, Bagsvaerd, Denmark, 100 U/mL) with pre-meal insulin Aspart (NovoRapid® FlexPen®, 100 U/mL). Figure 1 shows a summary of the study process.
3.2. Patients and Data Collection
Children with T1DM who were referred to the Children's Medical Center of Tehran were selected. Diagnostic criteria were based on ISPAD Clinical Practice Consensus Guidelines (18). Included patients were children with T1DM diagnosed before the age of 6 years, with at least 1 year passed since their diagnosis to avoid honeymoon period variations. Considering the inclusion criteria, 10 individuals were excluded (four due to connection loss or the unwillingness of parents to continue detemir use, two because of detemir inaccessibility, one due to the initiation of treatment with metformin, one because of being diagnosed with monogenic diabetes, and two due to the disagreement of patient's physician with patient’s contribution to the study). Finally, 60 patients entered the study. Table 1 shows the baseline characteristic of the two groups. There was no significant difference in age or BMI between the two groups.
| Variables | Group 1 (n = 33) | Group 2 (n = 27) | P-Value |
|---|---|---|---|
| Gender (female), % | 55 | 59.0 | 0.712 |
| Age (mo) | 70.4 ± 26.0 | 68.8 ± 30.9 | 0.814 |
| BMI | 54.2 ± 26.3 | 45.7 ± 30.9 | 0.253 |
| Duration of disease (mo) | 26.5 ± 14.1 | 26.7 ± 17.3 | 0.954 |
a Values are expressed as mean ± SD unless otherwise indicated.
The exclusion criteria were the lack of proper disease control or any drug reactions leading to using other insulin types except detemir, inaccessibility to detemir insulin, any diagnosis other than T1DM raised during the study, or the use of any medications affecting blood glucose level. Patients who failed to be controlled properly and needed treatment modification were excluded from the final analysis for HbA1c or insulin dose comparison.
Medical records were utilized to collect baseline information, such as age, duration of disease, height, weight, and type of insulin administrated. Families were trained by one of the researchers in terms of using insulin detemir properly at home, and checking their blood glucose level (self-monitoring blood glucose, SMBG) at different times, including fasting, before lunch, 4 pm, before dinner, bedtime, and 3 am. Signs and symptoms of hypoglycemia were explained, and a 24-hour telephone connection was provided.
Hb1Ac, total daily insulin, and total daily detemir dose were recorded after the first month. Severe hypoglycemia, as well as diabetic ketoacidosis (DKA) or any situations leading to admission because of diabetes control, were considered as “complications”. Hypoglycemia was considered as blood glucose below 70 mg/dl with clinical symptoms or blood glucose below 50 mg/dl without any clinical symptoms. Severe hypoglycemia was defined as a seizure or loss of consciousness associated with blood glucose below 70 mg/dL.
3.3. Insulin Administration and Measurements
The study period was 4 months. The first month was devoted to patient and family education and insulin dose titration. Detemir insulin was prescribed in the mornings in group 1, and two equal doses were prescribed (mornings and evenings) for the patients of group 2. After the first month, there was no change in the overall insulin dose of patients, except in urgent cases. HbA1C was measured at the end of the first and fourth months as the main indicator of treatment effectiveness.
3.4. Ethical Approval and RCT Registry
Written informed consent was taken from parents. The RCT was reviewed and approved by the Iranian Registry of Clinical Trials on 2019-01-16. The registration reference is IRCT20181106041574N1. In addition, the ethical code of IR.TUMS.CHMC.REC.1397.014 was received from Tehran university of medical science.
3.5. Data Analysis
Descriptive statistics are presented as mean ± standard deviation for continuous variables, and frequency and percentage for categorical variables. Intention to treat analysis was used for the protection of randomization. Groups 1 and 2 were compared for continuous variables using the Student’s t-test, and categorical variables were compared using the chi-squared test or Fisher’s exact test, when appropriate. Intra-group comparisons between the first and fourth months were performed using paired t-test. All analyzes were conducted using the STATA Software Version 14 (Stata Corp LP Texas, TX, USA). P < 0.05 was considered statistically significant. The sample size was determined by assuming an alpha error of 0.05, a power of 80%, and the following equation:
4. Results
4.1. Total insulin Dose and HbA1c
Table 2 shows the total daily insulin dose, total daily detemir dose, mean bedtime blood glucose based on SMBG at the end of the fourth month, and HbA1c in the first and fourth months in the two study groups. There was no significant difference in HbA1c at the beginning (first month) between the two groups (P = 0.37). Moreover, in the fourth month, HbA1c was similar in both groups (P = 0.98). Although the total daily insulin dose was lower in group 2 than group 1, and the total detemir dose was lower in group 1 than group 2, the difference was not statistically significant. Group 1 had a slightly higher bedtime SMBG than group 2, but the difference was not significant. Paired t-test results showed no significant differences in the mean HbA1c in the fourth month compared to the baseline in both groups (Table 3).
| Variables | Group 1 | Group 2 | P-Value |
|---|---|---|---|
| Total insulin dose (U/Kg) | 1.067 ± 0.231 | 1.061 ± 0.239 | 0.93 |
| Total detemir dose (U/Kg) | 0.626 ± 0.16 | 0.650 ± 0.176 | 0.61 |
| Bedtime SMBG (mg/dL) | 209.6 ± 56.5 | 200.4 ± 57.3 | 0.58 |
| HbA1c, first month, % | 8.6 ± 1.2 | 8.9 ± 1.1 | 0.37 |
| HbA1c, fourth month, % | 8.5 ± 1.0 | 8.5 ± 1.1 | 0.98 |
| Variables | Mean ± SD | SE | 95% CI of the Difference | P-Value | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Group 1: HbA1c 4th month - HbA1c base | -0.09 ± 0.84 | 0.18 | -0.47 | 0.30 | 0.739 |
| Group 2: HbA1c 4th month - HbA1c base | -0.42 ± 1.19 | 0.25 | -0.94 | 0.09 | 0.109 |
4.2. Treatment Modification and Complications
Some patients from both groups were unable to achieve optimal control despite changing the insulin doses according to the reports of SMBG. With the decision of the medical team, including the researcher or patient's physician, detemir insulin administration in these patients was switched from one group to another group, or even detemir insulin was changed to another insulin type. These patients, who needed treatment modification were removed from the final analysis for HbA1c or insulin dose comparison at the end of the fourth month. Treatment modification was observed in 13 (39%) cases of group 1, and 4 (15%) individuals in group 2 (P = 0.02).
Among 13 patients in group 1, hypoglycemia during the day was found only in one case, but hyperglycemia (6 fasting, 6 fasting & evening) was the most common reason for changing the treatment. In group 2, one case due to DKA, and three patients due to hypoglycemia (one nocturnal, two diurnal) experienced treatment changes. Complications leading to admission entailed DKA in one patient of group 2, and severe (symptomatic) hypoglycemia in two patients of group 1. None of the other hypoglycemia reports were severe. Hospital Admissions and hypoglycemia were not significantly different between the two groups (Table 4).
| Outcome | Group 1 | Group 2 | P-Value |
|---|---|---|---|
| Treatment modification | 13 (39) | 4 (15) | 0.02 |
| Complications (leading to admission) | 2 (6) | 1 (4) | 0.67 |
| Hypoglycemia | 7 (21) | 5 (18) | 0.18 |
a Values are expressed as No. (%).
5. Discussion
Few studies have compared the effect of once and twice a day insulin detemir administration in children with T1DM. Therefore, findings for comparing the results of this research are limited. In addition, most investigations have been performed on patients older than 10 years and not on T1DM patients under 5 years old. Some participants with treatment changes during the study were excluded from the final analysis for disease control (HbA1c) or insulin dose comparison. Consequently, the results were compared between the two groups of patients who completed the study course without treatment change. The treatment modification rate was 39% in group 1, and 15% in group 2 (P = 0.02). However, there were no statistically significant differences in other complications, insulin dose, and hypoglycemia between the two groups.
5.1. Long-term Glycemic Control
Our findings showed that long-term glycemic control (HbA1c) was not different between the two groups after 3 months. Furthermore, changes in HbA1c were not significant in each group, compared to the baseline HbA1c, considering that at least 1 year had passed since the onset of diabetes in patients. However, the mean HbA1c in the first month in group 2 was higher than in group 1. Le Floch et al. compared the once-daily and twice-daily detemir administration in adult patients with T1DM. They concluded that although some patients may benefit from twice-daily doses, the once-daily dose is recommended because the differences in HbA1c were not significant between the two groups (19). Nimri et al. found no significant difference in HbA1c levels between the two groups (15). In their study, all patients initially received once-daily detemir, and they switched to twice-daily dose when once-daily detemir could not achieve proper fasting blood sugar, or if hypoglycemia occurred due to increased insulin dose. However, in the present study, all patients were divided into two groups of once-daily or twice-daily injections. Therefore, it has been proposed not to differentiate HbA1c based on the type of treatment method (once- vs. twice daily) both in this study and in similar studies.
5.2. Insulin Dose
In our study, the mean daily insulin dose in the twice-daily group was slightly lower than in the once-daily group, albeit without statistical significance. Despite the differences in the age groups of the studied patients, this finding is consistent with the results of Le Floch et al. (19). In our patients, detemir injection in the once-daily group was in the morning. As a result, one possible reason for the higher total insulin dose may be fasting and nocturnal hyperglycemia in this group, as observed in bedtime SMBG. In the study completed by Nimri et al., the results showed that the insulin dose in detemir twice daily was higher than in detemir once daily, which was not in line with the present research. This difference can be due to the changes in the treatment and hypoglycemia because of the study design (15).
5.3. Hypoglycemia
In our study, no difference was observed in terms of hypoglycemia occurrence between the two groups. However, the number of patients with hypoglycemia was higher in group 1 compared to group 2. Nimri et al. demonstrated that the hypoglycemia rate was higher in patients, who received detemir once daily compared to the twice-daily group. The findings of Nimri et al. were not in line with the current study, which may result from differences in the study design (15).
5.4. Treatment Modification
Treatment modification in group 2 was significantly lower than in group 1, indicating that once-daily detemir injection led to more often “treatment change”. In the research performed by Nimri et al., once-daily insulin detemir was switched to a twice-daily dose when it did not result in proper fasting blood sugar. Although in the latter investigation only 51% of patients eventually remained on the once-daily detemir, the researcher did not consider this remarkable (15). Other studies have not considered this change in treatment method important. However, in the present research conducted on young children, it seems that this factor should be considered in the selection of treatment methods because of a statistically significant difference.
5.5. Limitations
Further investigation on a larger sample sizes and evaluating the effect of insulin detemir in different age groups simultaneously is recommended.
5.6. Conclusions
According to the findings of the current study, the twice-daily injection did not change the HbA1c level in comparison with once-daily injection. However, the lower treatment modification rate in the twice-daily group was considerable in the assessed age group.