1. Background
Cancer mortality in pediatrics aged from 1 to 14 years is the most common cause of non-accidental death in the USA. More than 1,500 pediatrics per year are diagnosed with cancer in the United Kingdom (1, 2). The American Childhood Cancer Organization (ACCO) has reported that leukemia (30%), brain and CNS tumors (26%), and lymphomas (8%) were the three most common types of cancer in children (2). Radiotherapy is the process of using high and homogeneous doses of ionizing radiation to treat various malignant diseases (3). Radiotherapy aims to render the highest possible radiation dose to the tumor while sparing as much of the normal healthy tissue as possible (4).
Radiotherapy may be used as a multi-purpose therapy, adjuvant or neoadjuvant therapy, curative (prevention of organ failure), or palliative purposes (5, 6). For performing radiation beams properly, it emphasizes patient immobilization and controlling patient movement during radiotherapy treatment (7). This process is often concise and needs anesthesia to prevent anxiety and provide motionlessness during radiotherapy in pediatrics. Radiotherapy may bring about various complications, including death, hypothermia, airway obstruction, hypoventilation, aspiration, hypovolemia, anaphylaxis, and procedure-related complications.
Some studies reported non-medication techniques such as watching cartoons (8), hand-held video gameplay (9), and the presence of clown doctors (10) to relieve anxiety and decrease the need for anesthesia. Many studies have presented several anesthesia techniques and associated complications for performing pediatrics conventional radiation therapy (6, 7, 11, 12). Radiotherapy in pediatrics with brain tumors under anesthesia presents several challenges due to physiology, tumor site, and higher risk for respiratory depression and life-threatening conditions.
When treated with radiotherapy and chemotherapy, pediatricians with brain tumors experience potential neurologic complications, especially if done frequently under anesthesia. Some complications are neurocognitive changes, leukoencephalopathy; acute neuroendocrine dysfunction arising from irradiation of the hypothalamus or pituitary (chronic dysfunction is associated with younger age at the commencement of treatment); brain lesions due to raised intracranial pressure, and Para-neoplastic syndromes like Eaton–Lambert may occur (7, 11, 12). Thus, understanding these complications is very important for radiation therapy and anesthesia teams.
The aim of anesthesia in pediatrics with brain tumors is to provide adequate analgesia, sedation, and anxiolytics, control unwanted motor activities while performing a diagnostic or therapeutic procedure to enable a rapid return to the baseline level of consciousness, and to reduce risks of adverse events related to anesthesia (13).
It is widely stated that children under three cannot understand the procedure and cooperate during radiotherapy, especially if it is a complicated and prolonged treatment. Consequently, the use of anesthesia or sedation is often required (5, 14-16). However, no studies have investigated the neurologic complications associated with anesthesia in pediatrics treated with radiotherapy under anesthesia.
2. Objectives
This study aimed to evaluate the neurologic complications associated with anesthesia in pediatrics treated with radiotherapy under anesthesia. This results of this study would help identify and manage the factors involved in these patients' neurologic complications.
3. Methods
In this retrospective cross-sectional study, we enrolled 133 pediatrics patients with brain tumors who were referred to Omid Charity Hospital and Imam Khomeini University Hospital and needed anesthesia for performing radiotherapy by the census from 2014 to 2020. The data collection tool was a researcher-made checklist containing age, sex, weight, associated diseases, and applied treatments.
3.1. Inclusion and Exclusion Criteria
Inclusion criteria were all pediatrics with brain tumors treated with radiotherapy under anesthesia.
Exclusion criteria were pediatrics with other malignancies (the exception for brain tumors) and pediatrics who had done part of their treatment in other radiotherapy centers. Also, pediatrics with congenital diseases, such as thalassemia, hemophilia, cardiac, and other organ anomalies, Down syndrome, and cerebral palsy were excluded from the study.
Four anesthesia techniques were used in this study, including sedation with midazolam, sedation with midazolam + propofol lidocaine, ketamine intramuscular, and ketamine + midazolam.
Neurologic complications and complications associated with anesthesia in pediatrics undergoing radiotherapy were recorded in a researcher-made checklist. The validity and reliability of the checklist were confirmed by experts and using Cronbach's alpha coefficient (α ≥ 0.7). Major complications were defined as the following: Stroke, lethal arrhythmias, cardiopulmonary resuscitation, tachyphylaxis, aspiration, laryngospasm, and advanced airway interventions such as urgent endotracheal intubation, unexpected admission to the hospital, and bronchospasm. However, oxygen desaturation to 5% of the base value, nausea, vomiting, hypotension, apnea ≥ 10 (Second), and delay in consciousness were classified as minor complications. Neurologic complications were defined as seizures, headaches, irritability, lethargy and drowsiness, and visual disorder.
Standard anesthesia monitoring methods such as electrocardiography, pulse oximetry, and non-invasive blood pressure were placed. Moreover, contra-indicate anesthetic drugs were considered for using in child-specific conditions.
Descriptive statistics were used to show the frequency and mean. After data collection, utilizing a reliable checklist, chi-square statistical test analyzed pair-wise relationships among categorical risk factors (tumor side and applied anesthetic drugs, anesthesia complication, neurologic complications, and performed chemotherapy). We also analyzed the number of anesthesia-related complications for procedure duration: 5 min, 5 - 10 min, and 10 - 15 min. The SPSS-25 software was used for statistical analysis, and statistical values less than 0.05 were considered significant (P < 0.05).
3.2. Ethical Approval and Patient Consent
Urmia University of Medical Sciences (IR.UMSU.REC.1399.097) approved the study.
4. Result
The patients were between 1 - 8 years old, and 3,208 inductions of anesthesia were conducted for daily radiotherapy (mean per patient was 24.12). Patients' mean age was 5.13 ± 2.24, the demographic characteristics of pediatrics are presented in Table 1.
| Variables | Values |
|---|---|
| Age (y) | 5.13 ± 2.24 |
| Gender | |
| Male | 56 (42.11) |
| Female | 77 (57.89) |
| Weight (kg) | 14.62 ± 6.17 |
aValues are expressed as No. (%) or mean ± SD.
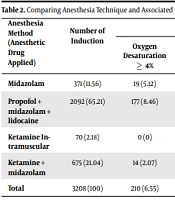
Minor anesthesia complications included oxygen desaturation to 5% of base value observed 210 times, nausea and vomiting 46 times, hypotension 58 times, apnea 25 times, and delay in consciousness return 38 times. Major complications such as stroke, lethal arrhythmia, tachyphylaxis, cardiopulmonary resuscitation, aspiration, laryngospasm, urgent intubation, unplanned admission to the hospital, and bronchospasm were not observed. There was a significant relationship between the tumor’s location, duration of the radiotherapy period, and ensured anesthesia prolongation and neurologic complications (P < 0.05). Although anesthesia complications in this study were minor, some occurred due to the tumor effect on other vital organs and previous chemotherapy (Tables 2-5).
| Anesthesia Method (Anesthetic Drug Applied) | Number of Induction | Anesthesia Complication | Insufficient Motionless | Delay in Consciousness Return | P-Value b | |||
|---|---|---|---|---|---|---|---|---|
| Oxygen Desaturation ≥ 4% | Nausea and Vomiting | Hypotension | Apnea ≥ 10 (S) | |||||
| Midazolam | 371 (11.56) | 19 (5.12) | 12 (3.23) | 0 (0) | 2 (0.54) | 13 (3.5) | 0 (0) | 0.011 |
| Propofol + midazolam + lidocaine | 2092 (65.21) | 177 (8.46) | 0 (0) | 56 (2.68) | 23 (1.1) | 0 (0) | 0 (0) | 0.038 |
| Ketamine Intramuscular | 70 (2.18) | 0 (0) | 2 (2.86) | 2 (2.86) | 0 (0) | 13 (18.57) | 31 (44.29) | 0.016 |
| Ketamine + midazolam | 675 (21.04) | 14 (2.07) | 32 (4.74) | 0 (0) | 0 (0) | 24 (3.55) | 7 (1.04) | 0.024 |
| Total | 3208 (100) | 210 (6.55) | 46 (10.83) | 58 (5.54) | 25 (1.64) | 50 (25.62) | 38 (45.33) | |
aValues are expressed as No. (%).
bAnalyzed by chi-square test. The chi-square test showed a significant difference between the four groups in terms of insufficient motionless, delay in consciousness return, oxygen desaturation ≥ 4%, nausea and vomiting, hypotension, and apnea ≥ 10 (S).
| Tumor side | Frequency (%) | Number of Anesthesia Complications (%) | P-Value a |
|---|---|---|---|
| Cerebrum | 56 (42.11) | 103 (30.38) | 0.014 |
| Mide brain (orbit, optic glioma & retinoblastoma) | 28 (21.05) | 94 (27.73) | |
| Brainstem | 28 (21.05) | 90 (26.55) | |
| Brain and spine | 21 (15.79) | 52 (15.34) | |
| Total | 133 (100) | 339 (100) |
aAnalyzed by chi-square test. The chi-square test showed a significant difference between the tumors’ location with anesthesia complications in pediatrics.
| Procedure Duration | Frequency (%) | Number of Anesthesia Complications (%) | P-Value a |
|---|---|---|---|
| 5 min | 63 (47.37) | 64 (18.88) | 0.032 |
| 5 - 10 min | 42 (31.58) | 110 (32.45) | |
| 10 - 15 min | 28 (21.05) | 165 (48.67) | |
| Total | 133 (100) | 339 (100) |
aAnalyzed by chi-square test. The chi-square test showed a significant relationship between the procedure duration and anesthesia complications in pediatrics with brain tumors.
| Neurologic Complications | Frequency (%) | P-Value a |
|---|---|---|
| Seizure | 24 (7.84) | 0.092 |
| Nausea and vomiting | 42 (13.73) | |
| Headaches | 72 (23.53) | |
| Irritability | 84 (27.45) | |
| Lethargy and drowsiness | 54 (17.65) | |
| Visual | 30 (9.8) | |
| Total | 306 (100) |
aAnalyzed by chi-square test. The chi-square test showed there was no significant relationship between neurologic complications.
4.1. Anesthesia Techniques and Associated Complications
The total anesthesia-related complications incidence was 10.57%, which was observed 339 times among 3,208 inductions. The most common complication was oxygen desaturation ≥ 4%, mainly in patients who were anesthetized by propofol + midazolam + lidocaine drugs (incidence = 8.46%). Furthermore, intramuscular ketamine and propofol + midazolam + lidocaine combination showed more hypotension occurrence with 2.86% and 2.68% incidence, respectively. Also, the prevalence of apnea ≥ 10 (Second) was higher in the propofol + midazolam + lidocaine combination (1.1%) than the other drugs. According to chi-square test, these differences were statistically significant (P < 0.05, Table 2). Nonetheless, nausea, vomiting, insufficient motionlessness, and delay in conscious return were not observed with the propofol + midazolam + lidocaine technique. This difference was statistically significant (P = 0.038, Table 2).
Delay in consciousness return in the pediatrics who were anesthetized with intramuscular ketamine technique was more than other techniques (prevalence 31 times = 44.29%), and this difference was statistically significant (P = 0.016, Table 2).
Insufficient motionless occurred in intramuscular ketamine, ketamine + midazolam, and midazolam alone with the frequency of 18.57% (the most significant), 3.55%, and 3.5%, respectively. The chi-square test showed this difference was statistically significant (P < 0.05, Table 2).
4.2. Tumor Location in Pediatrics with Brain Tumor
There was a significant statistical relationship between the tumors' location and anesthesia complications. Pediatrics who suffered from midbrain tumors (orbit, optic glioma, and retinoblastoma) and brainstem tumors experienced more anesthesia complications than other pediatrics with other types of brain tumors (P = 0.014, Table 3).
4.3. Procedure Duration and Anesthesia Complications in Pediatrics with Brain Tumor
There was a significant statistical relationship between the procedure duration and anesthesia complications in pediatrics with brain tumors. Pediatrics who were anesthetized for long-term radiotherapy procedures had a higher prevalence of anesthesia complications (P = 0.032, Table 4).
4.4. Neurologic Complications in Pediatrics with Brain Tumor
Headache and irritability were the most common neurologic complications in this study. There was no significant statistical relationship between neurologic complications associated with anesthesia in pediatrics treated with radiotherapy under anesthesia (P = 0.092, Table 5). However, previous radiotherapy and chemotherapy influenced the prevalence of anesthesia complications (P = 0.041).
5. Discussion
Different drugs and diverse anesthesia techniques are used for pediatrics anesthesia in radiotherapy for ensuring immobility and sedation during this procedure, such as inhalation anesthesia (17); intramuscular and intravenous methohexital (18, 19); oral, intramuscular, and intravenous ketamine (19, 20); intravenous thiopental (21); intravenous meperidine; intravenous midazolam, and oral chloral hydrate (22). However, in recent years, propofol has become the standard anesthesia care for radiation therapy in children (23-25). In our study, we utilized multiple techniques and drugs. This study was conducted in a restricted area with specific demographic and genetic characteristics.
Sedation and anesthesia techniques were used in four stages: minimal sedation, moderate sedation, profound sedation, and general anesthesia with minor or major complications attached to them, especially in pediatrics with brain tumors. Several studies demonstrated anesthesia-related neurotoxicity when pediatrics undergo radiation therapy and receive multiple anesthesia (26-28).
There was no statistically significant relationship between age, gender, weight, and complications in this study. Same studies showed similar results (29, 30). In Owusu-Agyemang et al.’s study conducted on 340 pediatric undergoing radiotherapy, 9,328 inductions by TIVA (total intravenous anesthesia) were performed using propofol and, if necessary, dexmedetomidine and narcotic with complication incidence of 0.05%, including desaturation, seizure, laryngospasm, and bronchospasm (31).
In a prospective study, 26 institutions conducted 30,037 anesthetic/sedation inductions that demonstrated the overall incidence of complications of 5.3%. Cardiac arrest occurred once, oxygen desaturation below 90% for > 30 seconds occurred 471 times, and stridor and laryngospasm happened 13 times. Unexpected apnea, excessive secretions, and vomiting frequencies were 72, 125, and 142, respectively (32). Our study did not have any significant anesthesia complications, such as laryngospasm, aspiration, bronchospasm, stroke, arrhythmia, tachyphylaxis, and death. Minor complications were mainly related to the brain tumor's location, procedure duration, and previous chemotherapy that caused peripheral venous damage. Thus, the anesthesia technique was modified from intravenous approach to Intra muscle technique (IM ketamine).
Complications of ketamine-based anesthesia in children radiotherapy were 23 - 24% in previous studies, comprising inadequate movement control, sialorrhea, and slow recovery (7, 33). Furthermore, ketamine had the highest number of complications in a recently conducted review with a 24% overall rate (7, 33). Our study used ketamine intramuscular, and ketamine + midazolam was the leading cause of delay in consciousness return, insufficient motionlessness, nausea, vomiting, and patient discharge from the recovery.
Using intramuscular ketamine was due to the inability to obtain an intravenous line related to peripheral venous phlebitis due to chemotherapy. However, some studies stated that chemotherapy does not seem to add the appreciable risk of complications except neutropenic sepsis (22). On the other hand, using more recent medications such as propofol was lower. A study conducted 1,033 consecutive sedations in children radiotherapy; the overall complication rates were from 0.01 to 3.5% (22). Thus, based on the data available in this study, propofol + midazolam + lidocaine combination for anesthetizing children as sedatives have been associated with minor complications compared to other utilized anesthesia or sedation methods.
The difference between the incidence of complications in our study and other cited studies is the definition of desaturation, generally described as a decrease in the mean oxygen saturation of ≥ 4% that lasts for at least 10 seconds (apnea ≥ 10 (S)).
The present study showed a significant relationship between the tumor location, procedure duration, anesthesia, and neurologic complications. Anghelescu et al. found four factors that were significantly associated with the risk of complications: duration of the procedure, the total dose of propofol, anesthesia with propofol plus adjunct agents (as compared to propofol alone), and simulation (as compared to radiation treatment) (29).
5.1. Conclusions
Anesthesia is just one of many factors suggested to play a role in pediatric complications with a brain tumor. Anesthesia complications in the present study were minor and trivial. Furthermore, some of them were due to the tumor effect on other vital organs and previous radiotherapy or chemotherapy. To provide safe anesthesia, considering tumor effects on body organs and neurologic complications caused by it to reduce the anesthesia complications in pediatrics under radiotherapy can be very helpful and practical. The safest anesthetic needs to lie within the anesthesia provider, examining the patient and reviewing the patient’s past medical history. Consequently, the anesthesia team and radiation oncologists must keep up to date with the current knowledge and evidence in this area.
5.2. Suggestions
Conducting more research in this field and a combination of clinical knowledge and experience to generalize the results of evidence-based studies are suggested.