1. Background
Amino acids, proteinogenic units, and derivatives have critical functions in biological processes, such as protein synthesis and metabolic pathways. Enzyme deficiencies in amino acid metabolism can alter their physiological concentrations resulting in clinical symptoms usually not specific to a single disease. Therefore, it is essential to identify free amino acid concentrations in physiological specimens because one or few compounds may act as biomarkers for a single or a group of metabolic disorders, eg, phenylalanine for phenylketonuria (PKU), ornithine, citrulline, and argininosuccinic acid for urea cycle disorders, and allo-isoleucine and valine for maple syrup urine disease (MSUD) (1). Free amino acid concentrations are used in clinical trials and for diagnostic purposes for evaluating patients' nutritional status (2).
Congenital metabolic disorders are a complex and heterogeneous group of genetic disorders that originate from a defect in the metabolic pathway and lead to malfunctioning metabolism and/or accumulation of toxic intermediate metabolites (3). Congenital metabolic disorders can occur at any age, manifest with non-specific clinical signs, and have a complex diagnostic process. They are associated with serious outcomes in clinical practice leading to morbidity and mortality, particularly in pediatrics. Although each disease is individually rarely encountered, the cumulative incidence is reported to be higher than one in 800 (4).
Measurement of amino acids in dried blood spot (DBS) has been widely used to identify newborns with various congenital disorders of amino acid metabolism, including PKU and MSUD (5). Collecting human blood through heel or finger puncture and dripping on a filter paper to obtain dried blood samples for analysis dates back to the early 1960s when Guthrie first used dried blood samples to determine phenylalanine concentration for the diagnosis of PKU in newborns (6). It is emphasized that dried blood samples have many specimen-related advantages over conventional blood, plasma, and serum samples (7-9). This novel way of blood collection has led to neonatal screening and other clinical tests (7, 8, 10). Metabolic screening of newborns with a Tandem mass spectrometer (MS/MS) using dried blood samples was first used by Millington et al. (11) for the analysis of acylcarnitines and Chace et al. (12) for the analysis of phenylalanine and tyrosine. Along with accelerated development in MS technology in the last decade, MS/MS systems with substantially improved precision and selectivity began to be used for quantitative analysis of various molecules in dried blood samples for the diagnosis of various hereditary metabolic disorders, as demonstrated through numerous practices in neonatal screening (13-17).
Dried blood spot samples are suitable for the diagnosis of some congenital errors of the metabolism; however, they provide limited benefits in regular monitoring of amino acids. Monitoring of plasma amino acids is frequently required to assess the efficacy of treatment in patients with PKU, MSUD, and some urea cycle defects (5).
2. Objectives
This study aimed to analyze the amino acid levels in simultaneously sampled dried blood spots and plasma by the liquid chromatography-tandem mass spectrometry (LC-MS/MS) method.
3. Methods
The study used the data of 145 patients (17 from the pediatrics ward, 70 from the endocrinology ward, 11 from the neonatal unit, and 47 from the pediatric intensive care unit) who applied to our hospital and had simultaneously sampled plasma and dried blood spots between January 2017 and December 2018. Of the patients, 50 were female, and 95 were male. This research project was approved by the Health Sciences University Diyarbakır Gazi Yaşargil Training and Research Hospital Ethics Committee (No. 27.09.2019 - 341).
The study samples were analyzed in an LC-MS/MS System (Zivak Technologies, Turkey) device using the original kits routinely used in our laboratory. For dried blood samples, amino acids were extracted from blood samples dried with an organic solvent containing internal standards. The extracted analytes were derivatized with reagents. Derivatized amino acids were analyzed in the LC-MS/MS system. The isotope dilution method was used to compute the results. Concentrations of the analytes were calculated according to the given internal standard areas.
For plasma amino acid concentrations, the samples were drawn into tubes containing dipotassium ethylenediaminetetraacetic acid (Becton Dickinson Vacutainer Systems, USA). Amino acid concentrations were studied in plasma samples separated by appropriate centrifugation. Plasma amino acids were extracted from the samples by acidic extraction and deproteinization processes. The extracted analytes were derivatized with reagents. Derivatized amino acids were subjected to the volatilization process until they dried thoroughly. Then, they were dissolved again by adding reagents and analyzed in the LC-MS/MS system. The concentrations of standard solutions were plotted against the area of the standards. The signal value corresponding to each standard was included in the graphs to calculate the calibration curve. Unknown concentrations could be directly read from the function of the calibration curve.
The outcomes were alanine (Ala), arginine (Arg), citrulline (Cit), glutamic acid (Glu), glycine (Gly), isoleucine (Ileu), leucine (Leu), methionine (Met), ornithine (Orn), phenylalanine (Phe), tyrosine (Tyr), and valine (Val) amino acids, which are common in both methods. Since Leu and Ileu gave a total result in dried blood samples, a comparison was made by taking the sum of these two amino acids.
3.1. Statistical Analysis
Statistical analyses were done using SPSS and MedCalc package programs. The Kolmogorov-Smirnov test analyzed whether the data were distributed normally, and the Wilcoxon signed-rank sum test assessed the differences between the groups. A P-value < 0.05 was considered statistically significant. In addition, dried blood and plasma samples were compared using Bland Altman plots and Deming regression analysis.
4. Results
We used the data of 145 patients (50 females and 95 males) with amino acid concentrations simultaneously studied in dried blood and plasma samples. The mean age was 3.07 years (SD: 4.75, min: 0.01, max: 16.90) for females and 5.57 years (SD: 6.29, min: 0.01, max: 23.27) for males.
Table 1 illustrates the percentage coefficient of variation (CV) for all plasma and dried blood spot amino acids and their reference values. Amino acid concentrations of the patients are presented as mean ± SD, median, minimum, and maximum in Table 2. While there was a significant difference between dried blood samples and plasma samples for Phe, Tyr, Ala, Arg, Cit, Glu, Gly, Leu+Ileu, and Orn amino acid concentrations, no significant difference was determined for Met and Val amino acids (Table 2).
| Features | ALA | ARG | CIT | GLU | GLY | LEU+ILEU | ILEU | LEU | MET | ORN | PHE | TYR | VAL |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plasma | |||||||||||||
| Inter-assay CV% | 5 | 5.1 | 6.5 | 5.2 | 4.8 | - | 5.2 | 5 | 4.1 | 6.2 | 3.3 | 3.1 | 3.1 |
| Intra-assay CV% | 4.6 | 4.9 | 5.6 | 5.1 | 4.4 | - | 4.8 | 4.9 | 4.2 | 5.2 | 3.1 | 3.2 | 2.9 |
| < 24 monthsa | 139 - 474 | 29 - 134 | 9 - 38 | 31 - 202 | 111 - 426 | - | 31 - 105 | 48 - 175 | 11 - 35 | 20 - 130 | 28 - 80 | 26 - 115 | 83 - 300 |
| 2 - 17 yearsa | 144 - 557 | 31 - 132 | 11 - 45 | 22 - 131 | 149 - 417 | - | 30 - 111 | 51 - 196 | 11 - 37 | 22 - 97 | 30 - 95 | 31 - 106 | 106 - 320 |
| Dried blood spot | |||||||||||||
| Inter-assay CV% | 3.3 | 3.6 | 4.9 | 4.8 | 3.8 | 3.9 | - | - | 3.5 | 2.2 | 3.7 | 3.5 | 3.2 |
| Intra-assay CV% | 3.3 | 3.1 | 4.5 | 5.4 | 4.1 | 3.4 | - | - | 3.3 | 2.3 | 4.2 | 3.1 | 3.5 |
| < 4 days b | 424-633 | 5.6 - 14.7 | 13.8 - 23 | 485 - 687.4 | 300 - 414 | 511 - 703 | - | - | 10.7 - 17.7 | 124 - 185 | 50.3 - 70.1 | 74.1 - 114.4 | 89.1 - 179.2 |
| 6 -12 months b | 253.5- 355.8 | 14.2 - 23.5 | 18.5 - 25.6 | 194 - 306 | 146.3 - 251.6 | 305.4 - 440.1 | - | - | 9.4 - 21.9 | 69.3 - 122.5 | 40.5 - 62.7 | 52.6 - 85.5 | 118 - 160.3 |
| 1 -3 years b | 258.1- 406.2 | 12.7 - 25.1 | 21.9 - 31.8 | 201.2 - 283.8 | 168.2 - 332.2 | 282.9 - 436.2 | - | - | 7 - 18.5 | 70.2 - 137.1 | 49.3 - 72.3 | 58.3 - 93.3 | 120.1 - 205.3 |
| 3 -6 years b | 293 - 386.9 | 13.8 - 25.8 | 22.1- 31.8 | 214.8 - 262.3 | 157.1 - 229 | 275.3 - 396.1 | - | - | 8.2 - 15.6 | 82.4 - 111.3 | 47.1 - 84.2 | 50.1 - 91.3 | 103 - 174.4 |
| 6 - 12 years b | 221.6 - 307.8 | 15.4 - 20.7 | 24.4 - 28.8 | 211 - 258.7 | 257.1 - 305.9 | 343 - 472.5 | - | - | 17.1 - 22.6 | 68.7 - 88.4 | 53.3 - 66.2 | 62.3 - 84.3 | 128.4 - 171.4 |
a Reference values were obtained from Mayo Clinic Laboratories.
b Reference values were obtained from Uaariyapanichkul et al. (3).
| Amino Acids | Plasma Amino Acid Levels (µmol/L) | Dried Blood Amino Acid Levels (µmol/L) | P-Value a | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Median | Min | Max | Mean ± SD | Median | Min | Max | ||
| Ala | 384.75 ± 244.06 | 337.97 | 102.17 | 1.793.86 | 442.82 ± 215.45 | 418.24 | 1470 | 1.764.74 | < 0.001 |
| Arg | 517 ± 68.34 | 43.83 | 4.23 | 785.30 | 39.28 ± 44.11 | 34.44 | 69 | 513.52 | < 0.001 |
| Cit | 46.25 ± 212.48 | 236 | 0.45 | 2.456.35 | 40.20 ± 85.90 | 30.12 | 12.10 | 1500 | < 0.001 |
| Glu | 78.19 ± 496 | 67.72 | 6.81 | 270.87 | 226.34 ± 126.32 | 200.43 | 86.60 | 8680 | < 0.001 |
| Gly | 263.67 ± 121.91 | 2430 | 865 | 1.185.26 | 333.77 ± 174.33 | 2630 | 1420 | 935.75 | < 0.001 |
| Leu+Ileu | 172.84 ± 73.78 | 161.57 | 41.97 | 516.50 | 149.83 ± 50.56 | 1420 | 62.70 | 3420 | < 0.001 |
| Met | 42.58 ± 97.85 | 29.19 | 9.65 | 1.165.16 | 39.67 ± 76.56 | 29.10 | 13.80 | 924.45 | 0.751 |
| Orn | 1050 ± 68.82 | 88.59 | 6.28 | 548.12 | 1271 ± 84.56 | 95.40 | 40.60 | 5090 | 0.020 |
| Phe | 772 ± 89.14 | 562 | 3.83 | 7477 | 87.58 ± 97.17 | 670 | 32.25 | 963.37 | < 0.001 |
| Tyr | 909 ± 122.87 | 57.92 | 22.68 | 859.99 | 106.13 ± 126.29 | 75.61 | 35.90 | 950.10 | < 0.001 |
| Val | 190.41 ± 72.85 | 189.18 | 35.69 | 512.45 | 190.41 ± 59.31 | 181.11 | 66.90 | 4130 | 0.729 |
Abbreviation: SD, standard deviation.
a Significant at P-value < 0.05. Wilcoxon signed-rank sum test.
In 74 patients whose reference values (3, 18) were consistent with their age group, plasma and dried blood amino acid concentrations were categorized as low, normal, and high, which were matched. Dried blood spot Phe concentration was normal in four (36.4%) of 11 patients with high plasma Phe concentration, whereas dried blood Phe concentration was low in 13 (21.6%) and high in 14 (23.3%) of 60 patients with normal plasma Phe concentrations. Dried blood amino acid concentrations were normal in nine (36%) and low in six (24%) of 25 patients with high plasma Orn concentrations (Table 3).
| Amino Acids/ Plasma Levels | Dried Blood Levels | |||
|---|---|---|---|---|
| Low | Normal | High | Total | |
| ALA | ||||
| Low | 0 | 7 | 2 | 9 |
| Normal | 25 | 7 | 27 | 59 |
| High | 0 | 1 | 5 | 6 |
| ARG | ||||
| Low | 1 | 8 | 12 | 21 |
| Normal | 1 | 11 | 39 | 51 |
| High | 0 | 0 | 2 | 2 |
| CIT | ||||
| Low | 3 | 1 | 3 | 7 |
| Normal | 8 | 11 | 41 | 60 |
| High | 0 | 0 | 7 | 7 |
| GLU | ||||
| Low | 4 | 0 | 1 | 5 |
| Normal | 33 | 17 | 16 | 66 |
| High | 2 | 1 | 0 | 3 |
| GLY | ||||
| Low | 1 | 1 | 3 | 5 |
| Normal | 16 | 26 | 25 | 67 |
| High | 0 | 0 | 2 | 2 |
| LEU+ILEU | ||||
| Low | 4 | 0 | 0 | 4 |
| Normal | 63 | 0 | 0 | 63 |
| High | 6 | 1 | 0 | 7 |
| MET | ||||
| Low | 0 | 0 | 1 | 1 |
| Normal | 47 | 7 | 0 | 54 |
| High | 0 | 0 | 19 | 19 |
| ORN | ||||
| Low | 0 | 0 | 1 | 1 |
| Normal | 21 | 19 | 8 | 48 |
| High | 6 | 9 | 10 | 25 |
| PHE | ||||
| Low | 0 | 1 | 2 | 3 |
| Normal | 13 | 33 | 14 | 60 |
| High | 0 | 4 | 7 | 11 |
| TYR | ||||
| Low | 2 | 0 | 0 | 2 |
| Normal | 21 | 29 | 16 | 66 |
| High | 0 | 1 | 5 | 6 |
| VAL | ||||
| Low | 0 | 2 | 0 | 2 |
| Normal | 1 | 30 | 38 | 69 |
| High | 0 | 0 | 3 | 3 |
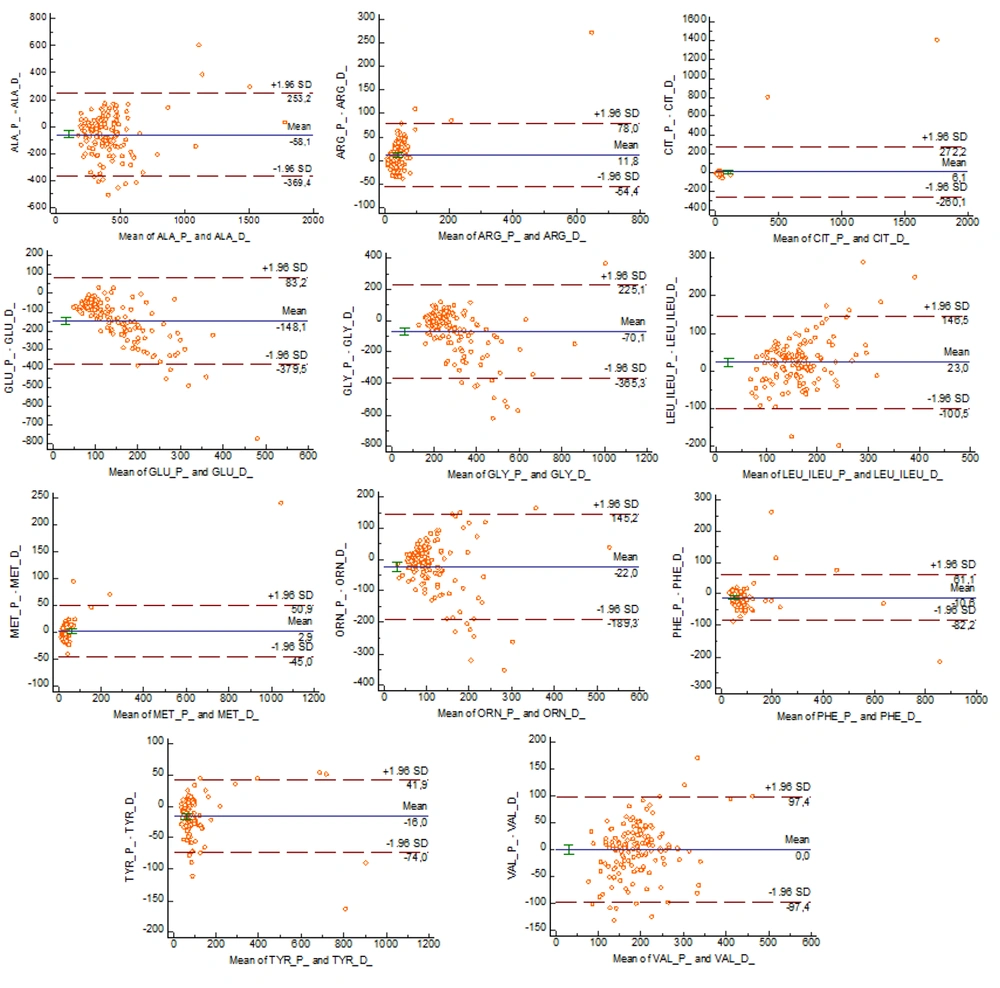
The mean differences between plasma and dried blood spot concentrations in Bland-Altman analysis were as follows: Ala (-58.1), Arg (11.8), Cit (6.1), Glu (-148.1), Gly (-70.1), Leu+Ileu (23), Met (2.9), Orn (-22), Phe (-10.6), Tyr (-16.0), and Val (0.0). Accordingly, while plasma and dried blood spot samples showed similar results for Val, the plasma Ala, Glu, Gly, Orn, Phe, - Tyr concentrations were lower, and Arg, Cit, Leu+Ileu, - Met concentrations were higher (Figure 1).
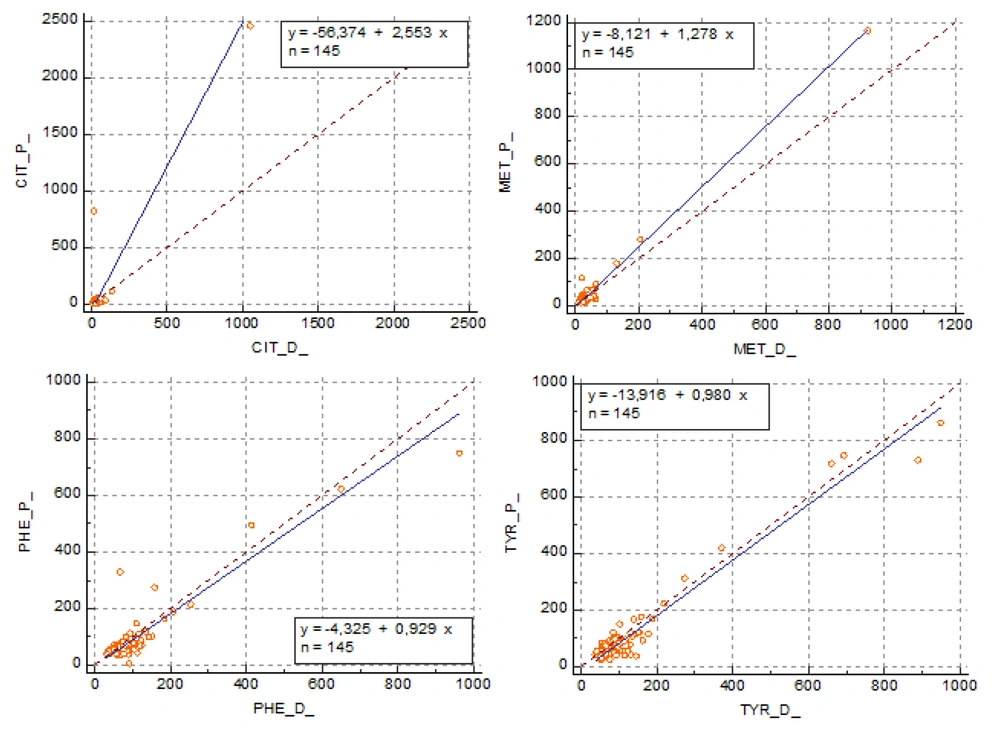
In Deming regression analysis, the confidence interval encompassed 1 for the slope and 0 for the intercept for Cit, Met, Phe, and Tyr. For other amino acids Ala, Arg, Glu, Gly Leu+Ileu, Met, Orn, and Val, the confidence interval did not include 1 and 0 values for the slope and intercept, respectively (Table 4). According to these data, Cit, Met, Phe, and Tyr amino acid concentrations appeared to be consistent in plasma and dried blood samples (Figure 2).
| Amino acids | Equation | Intercept (95% CI) | Slope (95% CI) | r |
|---|---|---|---|---|
| Ala | y = -94.8628 + 1831 x | -94.8628 –(189.9381 to 0.2125) | 1831 (0.8463 to 1.3199) | 0.77 |
| Arg | y = -8.7445 + 1.5229 x | -8.7445 (-22.6027 to 5.1136) | 1.5229 (1.1541 to 1.8917) | 0.91 |
| Cit | y = -56.3737 + 2.5530 x | -56.3737 (-10015.1099 to 9902.3624) | 2.5530 (-297.6161 to 302.7221) | 0.93 |
| Glu | y = -15.7609 + 0.4151 x | -15.7609 (-83.6542 to 52.1325) | 0.4151 (08597 to 0.7442) | 0.36 |
| Gly | y = 87.9261 + 0.5265 x | 87.9261 (-27.2085 to 203608) | 0.5265 (0.1419 to 0.9112) | 0.53 |
| Met | y = -8.1214 + 1.2785 x | -8.1214 (-18434 to 1.8005) | 1.2785 (0.9857 to 1.5713) | 0.99 |
| Leu+Ileu | y = -43.3491 + 1.4429 x | -43.3491 (-126.6931 to 39.9949) | 1.4429 (0.8466 to 2393) | 0.54 |
| Orn | y = 59.5131 + 0.3581 x | 59.5131 (19.9630 to 99633) | 0.3581 (-002288 to 0.7186) | 0.40 |
| Phe | y = -4.3254 + 0.9288 x | -4.3254 (-35.4172 to 26.7664) | 0.9288 (0.5177 to 1.3399) | 0.93 |
| Tyr | y = -13.9163 + 0.9800 x | -13.9163 (-26.9470 to -0.8855) | 0.9800 (0.8326 to 1.1274) | 0.97 |
| Val | y = -63.7035 + 1.3346 x | -63.7035 (-111.6221 to -15.7850) | 1.3346 (1756 to 1.5935) | 0.74 |
4. Discussion
Treatment of amino acid metabolism disorders allows avoidance of the toxic effects of dietary proteins and provides adequate protein intake for normal growth and development. For this purpose, specific low-protein or low-amino acid diets are established. Patients with amino acid metabolism disorders require close monitoring of protein intake for appropriate growth that changes with age, development, and other factors (5). Volume/size and quality of DBS and hematocrit (Hct) can significantly affect the analytical results of amino acids and, therefore, may have important effects in monitoring hereditary metabolic disorders (19, 20). Within this context, detection and monitoring of plasma amino acid concentrations are of critical importance.
Hct can substantially change according to age and gender, as well as the consequence of hydration status and because of diseases. Therefore, patient outcomes can show significant variations, and notably, DBS samples may not be the primary options for analysis in certain situations (21). Although neonatal screening programs are performed by MS/MS method in the USA, Canada, and many European countries, they are still not effectively performed in some Middle Eastern, African, and Latin American countries (22, 23). Different neonatal screening programs are implemented worldwide. In Turkey, however, phenylketonuria is included in amino acid screening programs where the Phe level is studied by the fluorescent immunoassay method (24).
Gregory et al. (25) detected a significant difference between Phe concentrations in plasma analyzed by an HPLC amino acid analyzer and dried blood analyzed by MS/MS, reporting that amino acid concentrations were 15% lower in dried blood than in plasma. They suggested that one should be careful about the measurement method of plasma phenylalanine concentrations for dieting. On the contrary, Phe and Tyr concentrations in the present study were significantly higher in dried blood than in plasma. Although studies comparing amino acid concentrations in plasma and dried blood are limited, clinicians must keep the concentration differences in mind during patient follow-up. Kazanasmaz et al. found similar results for Phe, Tyr, and Phe/Tyr in plasma and dried blood samples of 224 patients. In this study, the specimens were analyzed by the LC-MS/MS method, reporting that dried blood samples give results with clinically adequate specificity and sensitivity (26). Unlike the study mentioned above, the present study found a significant difference between plasma and dried blood samples in Phe and Tyr amino acids. The mean Phe and Tyr concentrations were higher in dried blood samples.
Groselj et al. (27) compared amino acids in plasma measured by an HPLC amino acid analyzer and dried blood measured by MS/MS, finding that dried blood showed lower concentrations by 26.1% for Phe and 15.5% for Try. They stated that the differences could be due to the methods used to diagnose and clinical monitoring of hyperphenylalaninemia patients. Significant differences between plasma and dried blood Phe and Tyr might have resulted from the differences in specimens or methods, which should be considered in the patient diagnosis and follow-up.
In Bland Altman's analysis, the mean difference was the highest at 148.1 for Glu, 70.1 for Gly, and 58.1 for Ala. Plasma and dried blood amino acid concentrations were evaluated in 74 patients with matched reference values. Dried blood amino acid concentrations were normal in four (36.4%) of 11 patients with high plasma Phe concentrations. The high mean differences between plasma and dried blood samples and inconsistencies between reference intervals for amino acids indicate that there may be errors during clinical decision-making. Although Wilcoxon signed-rank sum test revealed no significant difference between Val and Met amino acids, the samples were not compatible in terms of method consistency according to Deming regression. Nevertheless, Deming regression revealed that plasma and dried blood samples were compatible for Cit, Met, Phe, and Tyr amino acids.
The present study showed that amino acid concentrations showed significant differences between plasma and dried blood samples. Therefore, as comparative studies about amino acids are lacking, there may be specimen-related or methodological differences for patients, which should be considered in clinics. We think that it would be appropriate to follow plasma amino acids in order not to ignore the differences between the samples. The lack of clinical follow-ups in this study could be a significant limitation. In addition, further studies may consider the potential biochemical and clinical variables affecting plasma and dried blood spot amino acids in patients. Thus, more precise information can be obtained about the source of differences between the samples and their clinical usefulness.
Better outcomes are achieved in the identification of congenital metabolic disorders and amino acid concentrations with improving technology. Both methodological and specimen-related features influence amino acid concentrations, and DBS samples may cause errors even in screening programs. For this reason, clinicians and laboratories should consider that deviations in the specimens and systems might be crucial for the diagnosis and monitoring of metabolic diseases.