1. Background
Patent ductus arteriosus (PDA) device closure was first attempted by Porstmann et al. in 1967 (1). In recent years, percutaneous transcatheter closure of PDA has been a widely used treatment method. For the percutaneous transcatheter closure of the symptomatic PDA, Amplatzer duct occluders (ADOs) are commonly used (2). In neonates or patients with specific PDA morphology, PDA device closure has been difficult due to the device protrusion into the thoracic aorta (3-8). The right angle between the plug and disc causes the protrusion of the disc into the thoracic aorta because most PDA cases have an acute angle with the thoracic aorta. Therefore, clinical trials using modified or angled ADOs were reported (9, 10). These reports quoted from Mancini’s postmortem study in 1951 that resulted in an acute angle formed by PDA with the descending thoracic aorta of average 31.8° (11).
2. Objectives
However, there have been limited data about the angles of PDA since Mancini’s study in 1951, and new studies are required to develop better devices for PDA without complications, such as aortic obstruction. The present study measured the angles between PDA and descending thoracic aorta through angiography in a beating heart.
3. Methods
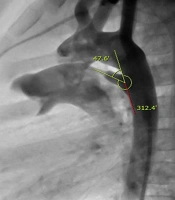
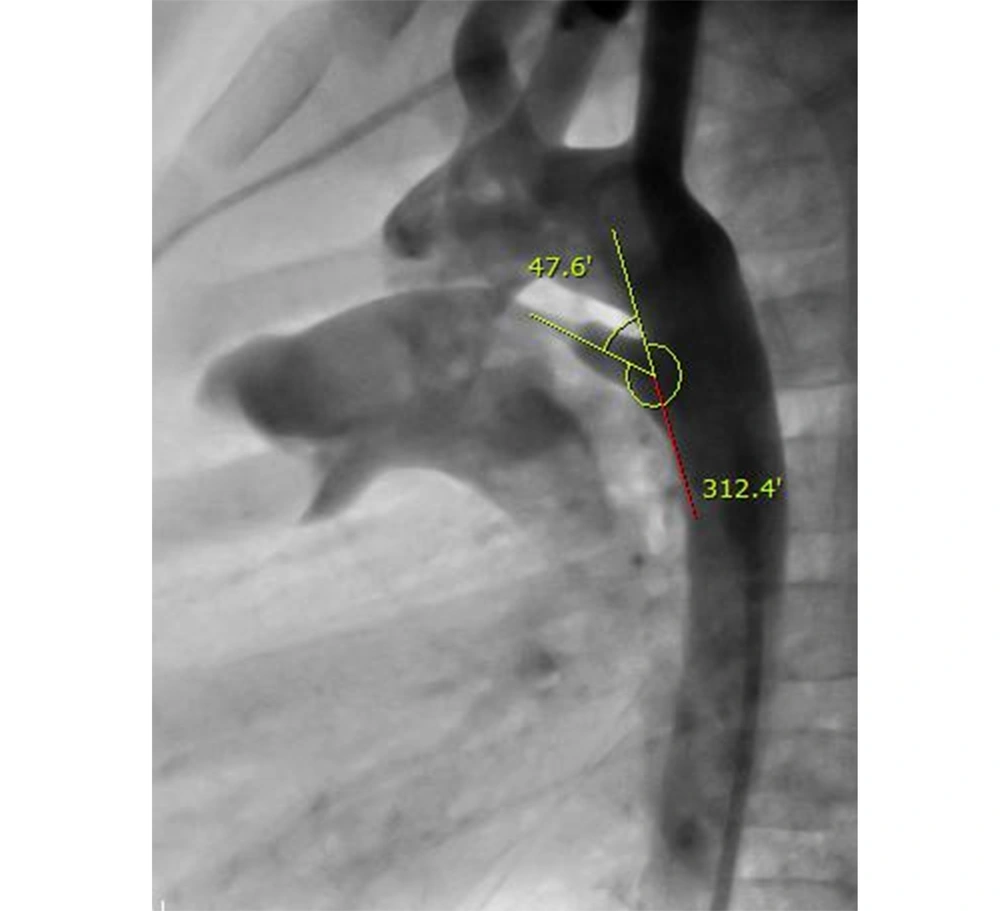
Within December 2008 to November 2016, 191 patients underwent percutaneous PDA occlusion using ADOs or coils. Among this group, one patient with a tortuous PDA was excluded from the study because it was impossible to measure the angle of tortuous PDA. Retrospectively, the PDA angle was measured by three cardiologists between the longitudinal axis of the descending thoracic aorta and the longitudinal axis of the PDA through an aortogram (Figure 1). The longitudinal axis of PDA was defined by a midline drawn across the mouth of the ductal ampulla to the narrowest diameter at the pulmonary end of the PDA. The patients were divided into three groups according to age (group A: under 1, group B: 1 - 6, and group C: over 6 years of age) and PDA morphology based on Krichenko’s classification (type A: conical PDA, type B: window PDA, type C: tubular PDA, type D: complex PDA, and type E: elongated PDA). Additionally, the average angle of PDA was also compared, respectively.
The continuous data were expressed as mean ± standard deviation. The student’s t-test and one-way analysis of variance were used for comparison of PDA angles among the groups. Statistical analysis was conducted using SPSS software (version 21.0). A p-value less than 0.05 was used to indicate the statistical significance. This study was approved by the Institutional Review Board and the Ethics Committee of Pusan National University Yangsan Hospital, Yangsan, South Korea. Furthermore, no informed consent was required for this study (05-2018-110).
4. Results
Of 190 study patients, 135 patients were female, and the median age of the patients was 7 years (range: 75 days to 60 years). The mean angle of PDA was 48.2 ± 12.0°. In the female group (n = 135), the mean angle of PDA was 48.0 ± 12.4°, and there was no statistical difference, compared to the male group (n = 55; 48.9 ± 11.4°) (Table 1). In 41 patients of group A (under 1 year of age), the mean angle determined by aortography was 47.5 ± 10.2°. In group B (1 - 6 years), 111 patients were included with a mean angle of 47.1 ± 11.2°. Furthermore, in group C (over 6 years of age), the mean angle was 52.3 ± 15.3° (Table 2). There were no statistical differences among the three groups classified by age.
| Population | Mean Angle of PDA |
|---|---|
| All (n = 190) | 48.2 ± 12.0° |
| Female (n = 135) | 48.0 ± 12.4° |
| Male (n = 55) | 48.9 ± 11.4° |
Mean Angle of Patent Ductus Arteriosus
| Groups Divided by Age | Mean Angle of PDA |
|---|---|
| Group A (< 1 years; n = 41) | 47.5 ± 10.2° |
| Group B (1 - 6 years; n = 111) | 47.1 ± 11.2° |
| Group C (> 6 years; n = 38) | 52.3 ± 15.3° |
Comparison of Patent Ductus Arteriosus Angles Among Groups Divided by Age
According to Krichenko’s classification, 137 (72.1%), 21 (11.1%), and 32 (16.8%) patients had PDA types A (conical), C (tubular), and E (elongated), respectively. In the group of type A, the mean angle was 48.4 ± 11.5°. In the group of type C, 21 patients were included with a mean angle of 49.7 ± 18.1°. Moreover, in the group of type E, the mean angle was 46.5 ± 9.1° (Table 3). There were also no statistical differences among the groups based on Krichenko’s classification. In this study, no patients had any acquired coarctation of the aorta after PDA device closure.
| Krichenko’s Classification | Mean Angle of PDA |
|---|---|
| Type A (n = 137) | 48.4 ± 11.5° |
| Type C (n = 21) | 49.7 ± 18.1° |
| Type E (n = 32) | 46.5 ± 9.1 ° |
Patent Ductus Arteriosus Angles According to Krichenko’s Classification
5. Discussion
The percutaneous transcatheter closure of PDA is now a gold standard treatment. The prevention of the protrusion of PDA device into the thoracic aorta is the main challenge with this technique, especially in small neonates or in specific PDA morphology with insufficient ampulla. In ADO I, the aortic disk might protrude into the aorta, causing obstruction in small children, particularly with specific PDA morphology that has insufficient ampulla (12). For the prevention of the protrusion of the PDA device into the thoracic aorta and ease of the transcatheter closure of PDAs with an insufficient ampulla, AGA Medical (USA) produced various prototype devices. For example, clinical trials using modified or angled ADO were reported (9, 10). These reports quoted from Mancini’s postmortem study in 1951 that resulted in an acute angle formed by PDA with the descending thoracic aorta of average 31.8° (11).
The ADO II was introduced to overcome the difficulty of the percutaneous transcatheter closure of PDA with insufficient ampulla, especially in small neonates (13-15). Numerous studies have reported that ADO II is appropriate for PDA device closure in challenging cases with ADO I (12, 14, 16-21). However, the protrusion of the ADO II device into the descending thoracic aorta also has been reported (5, 12, 19-21). The right angle between the plug and disc causes the protrusion of the disc into the thoracic aorta because most PDA cases have an acute angle with the thoracic aorta.
Currently, there have been limited data about the angles of PDA since Mancini’s study in 1951. Furthermore, there were several limitations in Mancini’s study. For example, the population was small, and Mancini measured the angle of PDA through autopsy in only neonates or stillbirth neonates. Therefore, new studies are required to develop better devices for PDA without complications, such as aortic obstruction.
This study measured the angle of PDA using the radiologic data from a beating heart in a sufficient population. The mean angle of PDA was 48.2°, higher than Mancini’s results. The present study had a broader range of age and a larger population than Mancini’s study that might be the reason for different results regarding the PDA angle. In this study, there were no statistical differences regarding the PDA angle among the groups classified by age and PDA morphology. This finding indicates that the mean angle of PDA might be applied without considering the age or PDA morphology. The authors are hopeful that the obtained data will help develop a better device for the percutaneous transcatheter closure of PDA.