1. Background
Respiratory distress syndrome (RDS) is the leading cause of neonatal mortality and morbidity, and it is mainly diagnosed with the help of clinical features and chest radiograph (1). Gestational age is the major risk factor, as 90% - 95% of the neonates aged between 22 - 24 week, 5% of those aged more than 34 week, and 1% of those aged 37 week will develop RDS. Furthermore, 25% of the neonates weighing 1,250 - 1,500 gr are at risk of RDS (1-3). The rest of the risk factors include maternal diabetes mellitus, white race, male gender, positive history of RDS in other siblings, diaphragmatic hernia, mutations of the genes in charge of synthesis of the surface proteins (SP) B and C, painless labor, and negative history of antenatal corticosteroid administration (1, 4).
The classic clinical manifestations include grunting, respiratory distress, nasal flaring, cyanosis, and requirement for supplemental oxygen. The typical radiographic findings include a diffused reticulogranular (ground glass) pattern and a bilateral air bronchogram (1). RDS worsens during the first few postnatal days and it is expected to resolve thereafter; though, the administration of surfactants can shorten this period (1). Since 1990, surfactants have been accepted as the preferred treatment because they are truly designed based on RDS pathophysiology (1). At the present time, two classes of surfactants are routinely used; artificial surfactants and natural surfactants, which are derived from porcine and bovine lung lavage extracts (5).
The administration of surfactants leads to a few adverse effects, including ductus arteriosus patency, pulmonary hemorrhage, and pulmonary edema. In contrast, the benefits include improved oxygenation, and decrease in mortality and morbidity, such as neurodevelopmental complications and air-leak syndrome (1, 6). Previous studies have demonstrated that natural surfactants are superior to artificial ones due to presence of the surface protein components (SP-B and SP-C) that can decrease the alveolar air-fluid interface (1, 5). Today, the repertoire of voguish natural surfactants comprises Survanta, Curosurf, Alveofact, Infasurf, and BLES (1, 7). BLES and Curosurf are the most widely available surfactants in Iran. Thus, this study attempts to compare them with respect to therapeutic characteristics and adverse effects.
2. Methods
This is a double-masked randomized clinical trial, which was performed at the NICU of Afzalipour tertiary medical center, Kerman, Iran, between November 2015 and November 2016. Study Protocol was approved in ethical research committee of Kerman University of Medical Sciences and parents of all neonates of the study signed the informed consent forms. A total of 240 neonates with established RDS were enrolled. They were divided into two major 120-member groups in such a way that the first group received Curosurf while the second one received BLES. Each major group was then equally divided into two sub-groups based on maternal history of betamethasone receipt.
The criteria for enrolling consisted of proven RDS through clinical and radiographic findings, and gestational age of 34 weeks or less. Clinical observations are defined based on the RDS score, which plays a pivotal role in the diagnosis of RDS. It comprises cyanosis, use of accessory respiratory muscles, auscultation quality of lung sounds through stethoscope, grunting, and respiratory rate number (8). The typical radiographic results included a diffused reticulogranular (ground glass) pattern and a bilateral air bronchogram (1). The neonates who conformed to these were chosen as the candidates for receiving the surfactant and were then enrolled in the study. The rest of the demographic data in both groups, such as gender, age, and severity of RDS, were matched.
The criteria for exclusion consisted of definite sepsis, congenital heart disease, genetic disorders, diaphragmatic hernia, congenital pneumonia, and persistent pulmonary hypertension (PPHN). Furthermore, any suspicion of asphyxia within the first day after birth convinced us to exclude that neonate from the study. At the beginning of the study, 252 neonates fulfilled the suggested inclusion criteria, but the condition of 12 ones exacerbated during the first three postnatal days, prior to allocation. This led to their exclusion from the study. Among these 12 excluded neonates, four developed sudden cyanosis that raised suspicion about congenital heart disease, five developed sclerema (pathologic skin hardening) and were suspected to have antenatal sepsis, and three suffered from convulsions, which could be due to asphyxia. The remaining 240 neonates were allocated accordingly. The neonates who fulfilled the criteria for enrollment were eligible to enter the study only after their parents gave full consent for the same. The recruited neonates were divided into principal groups of BLES and Curosurf administration. BLES and Curosurf were administered as 5 mL/kg (200 mg/kg) and 2.5 mL/kg (200 mg/kg) of dosage, respectively (9, 10).
Curosurf (poractant alfa) is available in 3-mL vials (80 mg/mL), manufactured by the Italian company Cheisi. It contains porcine lung lipid extracts, which is intratracheally administered. BLES (bovine lung lipid extract surfactant), which is slowly administered through the tracheal tube, is manufactured in Canada and is available in 5 mL vials (135 mg/5 mL). During surfactant administration, close cardiopulmonary monitoring must be done. All surfactant vials have to be preserved in refrigerator (2 - 8°C) and rewarmed prior to administration. Tracheal tube should be suctioned gently before administering the surfactant. The suction process should be delayed for at least six and two hours after administration of Curosurf and BLES, respectively. The indices, such as the OI (oxygenation index) of the first 12 hours after administration of surfactant, time needed for assisted ventilation or NCPAP (nasal continuous positive airway pressure), repetitive doses of surfactant, hospital stay, expenditure, the surfactant induced adverse effects (pulmonary hemorrhage or edema, intraventricular hemorrhage), and mortality were considered in comparing the two groups.
The OI equation is as follows: OI = FiO2 × Mpaw/PaO2.
The OI can be classified as good (OI < 5), medium (OI = 10 - 20), and poor (OI > 25) (11). The data was analyzed by SPS software, version 24. Quantitative and qualitative data were denoted by mean and standard deviation, respectively. The chi-square test and t-test were used to compare the qualitative and quantitative variables, respectively, with a significance of 0.05. The balanced block randomization was used to remove nuisance factors. This study complied with the ethical committee code of IR.KMU.REC.1395.839 and ID: IRCT2017021418994N3. All the parents were informed about the complete study process, the benefits of surfactants as well as its adverse effects. Only those who filled the consent forms were enrolled in this study.
3. Results
Among 240 neonates enrolled, 136 (56.7%) were boys and 104 (43.3%) girls. 74 (30.8%) and 166 (69.2%) were 28 - 30 and 30 - 34 weeks of gestational age respectively.
Ninety six (40%) of mothers had a positive history for diabetes mellitus in 39 (16.3%), hypertension in 39 (16.3%) and hypothyroidism in 18 (7.5%) (Table 1).
| Variable | No. (%) | Surfactant, % | Maternal Corticosteroid, % | ||
|---|---|---|---|---|---|
| Curosurf | BLES | Yes | No | ||
| Gender | |||||
| Girl | 1.4 (43.3) | 51a | 49a | 55.8a | 44.2a |
| Boy | 136 (56.7) | 49.3a | 50.7a | 45.6a | 54.4a |
| Route of delivery | |||||
| NVD | 54 (22.5)a | 40.7a | 59.3a | 46.3a | 53.7a |
| Cesarean section | 186 (77.5) | 52.7a | 47.3a | 51.1a | 48.9a |
| Weight, gr | |||||
| < 1000 | 49 (20.4) | 49a | 51a | 67.3b | 32.7b |
| 1000 - 1500 | 93 (38.8) | 53.8a | 46.2a | 45.2b | 54.8b |
| > 1500 | 98 (40.8) | 46.9a | 53.1a | 18.8b | 22.1b |
| Gestational age, weeks | |||||
| 28 - 30 | 74 (30.8) | 50a | 50a | 68.9b | 31.1b |
| 31 - 34 | 166 (69.2) | 50a | 50a | 41.6b | 58.4b |
| Maternal underlying disease | |||||
| No | 144 (60) | 54.9a | 45.1a | 53.5a | 46.5a |
| Yes | 96 (40) | 42.7a | 57.3a | 44.8a | 55.2a |
aP value > 0.05.
bP value < 0.05.
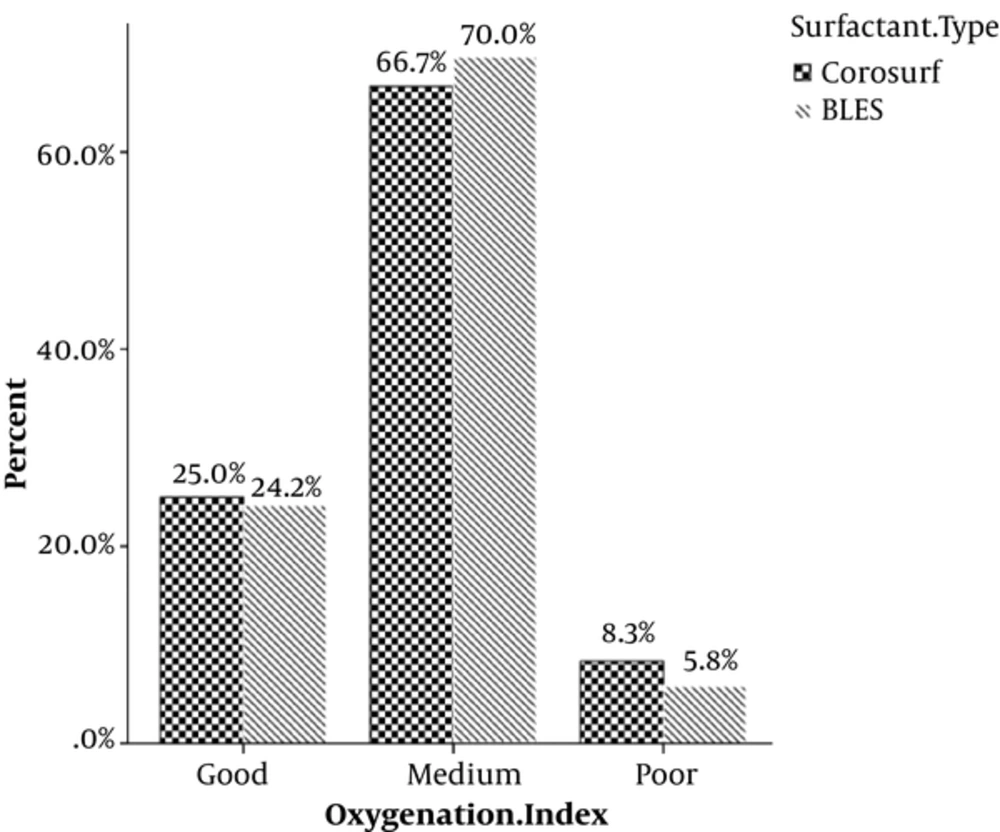
OI as the main outcome was associated with none of the two surfactant types (P = 0.725) (Table 2, Figure 1).
| Variable | No. (%) | Surfactant | ||
|---|---|---|---|---|
| Curosurf, % | BLES, % | P Value | ||
| Oxygenation index | 0.725 | |||
| Good | 59 (24.6) | 50.8 | 49.2 | |
| Medium | 164 (68.3) | 48.8 | 51.2 | |
| Poor | 17 (7.1) | 58.8 | 41.2 | |
| Need to ventilator, day | 0.625 | |||
| < 5 | 46 (19.2) | 54.3 | 45.7 | |
| 5 - 10 | 163 (67.9) | 47.9 | 52.1 | |
| > 10 | 31 (12.9) | 54.8 | 45.2 | |
| Need to NCPAP, day | 0.197 | |||
| < 3 | 24 (10) | 66.7 | 33.3 | |
| 3 - 6 | 85 (35.4) | 45.9 | 54.1 | |
| > 6 | 131 (54.6) | 49.6 | 50.4 | |
| Repetitive doses of surfactant | 0.794 | |||
| Yes | 102 (42.5) | 51 | 49 | |
| No | 138 (57.5) | 49.3 | 50.7 | |
| Duration of hospitalization, weeks | 0.953 | |||
| < 2 | 56 (23) | 48.2 | 51.8 | |
| 2 - 4 | 160 (66.7) | 50.6 | 49.4 | |
| > 4 | 24 (10) | 50 | 50 | |
| Mortality | 0.085 | |||
| Yes | 24 (10) | 66.7 | 33.3 | |
| No | 216 (90) | 48.1 | 51.9 | |
| Adverse effects | 0.62 | |||
| Yes | 45 (18.8) | 46.7 | 53.3 | |
| No | 195 (81.3) | 50.8 | 49.2 | |
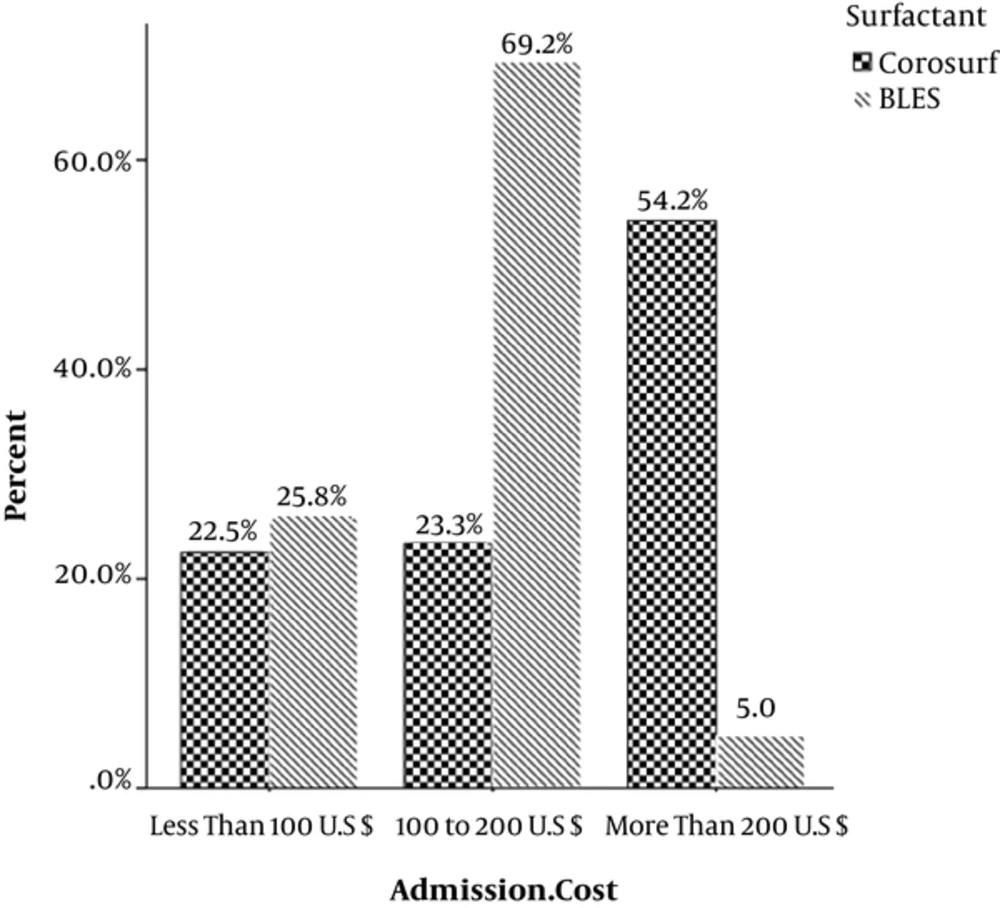
| Expenditure, U.S dollar | 0.000 | |||
| < 100 | 58 (24.2) | 46.6 | 53.4 | |
| 100 - 200 | 111 (46.3) | 25.2 | 74.8 | |
| > 200 | 71 (29.6) | 91.5 | 8.5 | |
The aforementioned indices, namely time of need to ventilator, time of need to NCPAP, repetitive doses of surfactant, hospitalization time, mortality and adverse effects of surfactants did not associate with the type of applied surfactant.
Mortality as a particular outcome was studied. Totally 24 (10%) neonates died during this trial; in which 16 belonged to Curosurf and 8 to BLES group. The difference was not significant (P = 0.085) (Table 2). Overall, pulmonary hemorrhage was the only adverse effect induced by surfactant, which was seen in 45 (18.8%) neonates. There was no significant difference between the two groups (P = 0.62) (Table 2).
Expenditure was the latest surveyed index that was significantly less in the BLES group than the Curosurf group (P = 0.00) (Table 2, Figure 2).
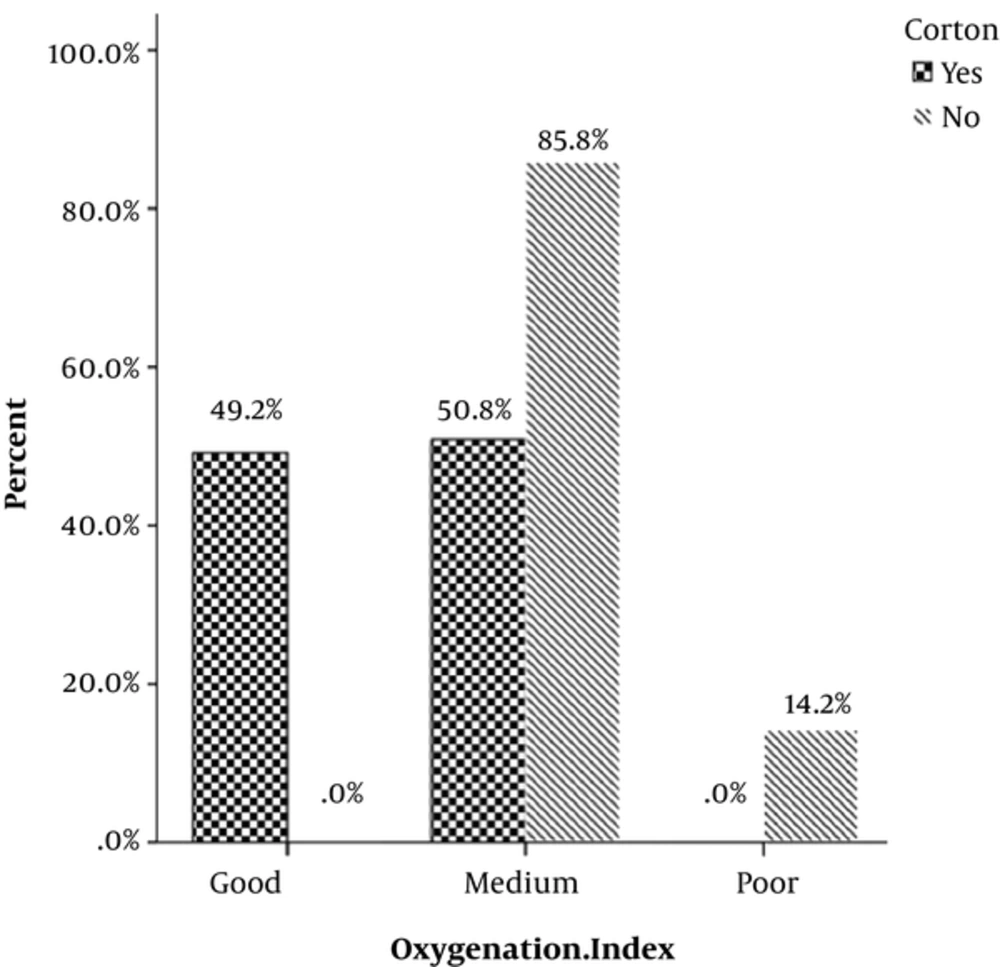
OI was associated with antenatal administration of betamethasone (P = 0.00) (Table 3, Figure 3).
| Variable | Maternal Corticosteroid | ||
|---|---|---|---|
| Yes, % | No, % | P Value | |
| Oxygenation index | 0.000 | ||
| Good | 100 | 0 | |
| Medium | 37.20 | 62.80 | |
| Poor | 0 | 100 | |
| Need to ventilator, days | 0.00 | ||
| < 5 | 100.00 | 0 | |
| 5 - 10 | 43.60 | 56.40 | |
| > 10 | 9.70 | 90.30 | |
| Need to NCPAP, day | 0.00 | ||
| < 3 | 54.20 | 45.80 | |
| 3 - 6 | 92.90 | 7.10 | |
| > 6 | 21.40 | 78.60 | |
| Repetitive doses of surfactant | 0.068 | ||
| Yes | 43.10 | 56.90 | |
| No | 55.10 | 44.90 | |
| Duration of hospitalization, weeks | 0.00 | ||
| < 2 | 100 | 0 | |
| 2 - 4 | 38.80 | 61.30 | |
| > 4 | 8.30 | 91.70 | |
| Mortality | 0.013 | ||
| Yes | 66.70 | 33.30 | |
| No | 46.20 | 53.80 | |
| Adverse effects | 0.013 | ||
| Yes | 66.70 | 33.30 | |
| No | 46.20 | 53.80 | |
| Expenditure, U.S. dollar | 0.000 | ||
| < 100 | 96.60 | 3.40 | |
| 100 - 200 | 31.50 | 68.50 | |
| > 200 | 40.80 | 59.20 | |
The indices including time of need to ventilator, time of need to NCPAP, repetitive doses of surfactant, time of hospitalization, expenditure, surfactant induced adverse effects and mortality were significantly associated with antenatal betamethasone (Table 3).
4. Discussion
As already mentioned, 240 neonates with gestational age of 34 weeks and less with proven RDS (based on clinical and radiographic findings) were recruited and then equally divided into two major groups. Members of first group received Curosurf and the second group received BLES. Each group was divided into two 60-member subgroups of receiving and not-receiving antenatal betamethasone. Among the studied variables, OI played pivotal role because it was proxy of blood gas analysis and ventilator parameters altogether. While comparing the groups, no association was found between OI and type of applied surfactant but on the contrary, OI was markedly associated with antenatal betamethasone.
The other surveyed variables such as time of need to ventilator, time of need to NCPAP, time of hospital stay, etc. were not significantly different comparing two major groups but they were associated with antenatal betamethasone.
Lam BC and co-workers compared Survanta and BLES studying 60 newborn infants with established RDS and weight of 800 - 1500 gr. OI within the first 12 hours after birth and surfactant induced adverse effects were determined as primary and secondary outcomes respectively. Both groups showed significant improvement (decrease) of OI but the BLES group’s OI was less. The other indices such as time of need to ventilator, time of need to NCPAP and time of hospital stay were not significantly different comparing the groups (9).
Ramanathan and associates compared Curosurf and Survanta studying 293 neonates with weight of 750 - 1750 gr and gestational age less than 25 weeks. A decrease in needed supplemental oxygen during first 36 hours of birth and surfactant adverse effects were estimated as primary and secondary outcomes respectively. Curosurf led to reduction of need to oxygen within first 6 hours of life, repetitive doses of surfactant and also adverse effects (10).
Saeidi and colleagues also investigated Curosurf and Survanta. They considered the needed FiO2 (fraction of inspiratory oxygen) within first 6 hours after birth and the time of need to ventilator as main variables. Both surfactants revealed nearly similar efficacy (12).
Crowther and colleagues clarified that antenatal corticosteroid could strongly alleviate the severity of RDS and need to oxygen supplementation in premature neonates. All types of applied surfactants had similar effect on the time of need to ventilator, this is comparable with our finding (13).
Lopez-Suarez and colleagues similarly specified that antenatal corticosteroid administration leads to unequivocal assuagement of RDS severity and also is associated with a clear decreased need to oxygen supplementation such as NCPAP and ventilator (14).
Smrcek and colleagues studied 365 neonates aged 28 - 34 weeks weighing less than 1500 gr. Those who received antenatal corticosteroid suffered from less severe RDS and mortality. Furthermore, the frequency of repetitive doses of surfactant declined. Type of applied surfactant was not associated with the variables (15).
Findings of our study showed that antenatal corticosteroid and type of applied surfactant did not influence mortality and repetitive doses of surfactant. The BLES receiving group paid fewer expenses because BLES was cheaper than Curosurf.
Regarding these findings, antenatal corticosteroid is still of utmost importance. In addition, based on almost similar efficacy of BLES and Curosurf; BLES can be a trustworthy product for surfactant therapy.
The major impetus of implementing this study was serious access limitations to surfactants in our NICU. Curosurf was more trendy and well-examined and also reliable. Even though, BLES had been recently engaged in our ward and also was more accessible and cheaper than Curosurf, therefore it made us to verify if BLES could be as capable as Curosurf for treatment of RDS. Consequently, there were no tendencies or orientations among authors to any of both types of surfactant.