1. Background
Obesity is a complex multifactorial condition characterized by excessive fat accumulation. It may play a role in pathophysiological changes such as inflammation, oxidative stress, endothelial dysfunction, and energy homeostasis disorder. Increased fat accumulation is associated with negative health consequences (1). Childhood obesity is an important public health problem of the 21st century with an increasing frequency. It affects approximately 25 - 30% of children globally (2). Non-alcoholic fatty liver disease (NAFLD), one of the problems caused by obesity, is seen in 22 - 52% of obese children (3). NAFLD is described as the collection of liver fat in excess of 5% in the lack of major alcohol consumption, viral infection, or any obvious etiology of the liver. Although there are many causes of fatty liver, an increase in the total of fatty acid coming to the liver and an increase in hepatic fatty acid synthesis are the main reasons (4).
Adipose tissue is considered to be an endocrine organ that secretes cytokines called adipokine (5). Dysregulation of adipokines stimulates systemic inflammation and contributes to obesity-related metabolic complications such as NAFLD, metabolic syndrome, insulin resistance, and cardiovascular disease (6). Omentin-1, also known as intelectin or endothelial lectin HL-1, is mainly secreted from the stromal vascular cells of the visceral adipose tissue. In addition, omentin-1 is secreted from intestinal paneth cells and endothelial cells. Omentin, which exists as two isoforms, is a protein of 313 amino acids primarily expressed in visceral adipose tissue rather than subcutaneous adipose tissue in humans; it is also expressed in epicardial adipose tissue (EAT) (7, 8). Omentin-1 is the main circulating isoform (9), and it has been indicated to increase insulin-stimulated glucose transport and Akt phosphorylation in adipocytes, suggesting that omentin-1 plays a part in cultivating insulin sensitivity (8). It has been reported that omentin-1 levels and omentin-1 gene expression in adipose tissue are lower in people with obesity, insulin resistance, or type 2 diabetes (9, 10). These data suggest that omentin-1 may play a protective role against obesity-related complications.
Although reduced omentin-1 levels have been associated with obesity in various studies, studies examining NAFLD and omentin-1 levels are limited (9, 11-15). Hence, the relationship between omentin-1 levels and NAFLD is still unclear (16-19). There is no study focusing on the relationship between NAFLD and omentin-1 levels, especially in the adolescent age group.
2. Objectives
The current study aimed to evaluate the circulating omentin-1 levels in obese adolescents with and without NAFLD and compare them with each other. Additionally, we aimed to investigate the relationship between omentin-1, body mass index (BMI), and some laboratory parameters.
3. Methods
3.1. Patients and Design
In this cross-sectional prospective cohort study, we included 56 obese adolescents (age range: 11 - 16 years) referred to the pediatric outpatient clinic (Istanbul Okmeydani training and research hospital, Turkey) between June and August 2020. As described by Mazıcıoglu et al., patients with a BMI ≥ 95th percentile, equivalent to a standard deviation score (SDS) of > 2, were considered to be obese based on their age and gender (20). Adolescents with grade 2 - 3 hepatosteatosis on upper abdominal ultrasonography (US) were considered to have NAFLD and were included in the NAFLD obese group (n = 28). Adolescents without US evidence of hepatosteatosis were included in the non-NAFLD obese group n = 28. The control group consisted of age- and gender-matched adolescents with no NAFLD and normal percentile BMI (n = 32).
BMI was determined by dividing weight in kilograms by the square of height in meters. The homeostatic model assessment for insulin resistance (HOMA-IR) was determined using the standard formula [insulin (IU)/L x glucose (mg/dL)/405] (21). Smokers, patients with chronic diseases, infection, inflammation, and thyroid disease, patients taking regular medication such as corticosteroids, and those with malignancy were excluded. Adolescents who had insulin resistance and were not obese according to HOMA-IR measurements were excluded from the healthy control group.
Subjects eligible for the study were informed about the study aims. All participants and their parents gave informed consent before participating in the study. The medical histories of the participants were recorded. Blood was drawn into a biochemistry blood tube. The blood tubes were first kept for 25 minutes and then centrifuged at 4000 rpm for 15 minutes. Sera were removed and stored at -80°C until analysis.
3.2. Omentin-1 Measurement
Omentin-1 levels were evaluated by a trading enzyme-linked immunosorbent assay (ELISA) in sera that were allowed to thaw at room temperature (Human Omentin-1, Elabscience Lot No: MPXGS3PF). The analytical measuring scale was 0.63 - 40 ng/mL for omentin-1. The lower finding cutoff was 0.38 ng/mL. The announced intra-assay and inter-assay CVs were < 4.79% and < 4.76%, respectively. Analysis was performed according to the manufacturer’s instructions.
3.3. Evaluation of Other Laboratory Tests
Glucose, urea, creatinine, aspartate aminotransferase (AST), alanine aminotransferase (ALT), C-reactive protein (CRP), total cholesterol, triglyceride, HDL-cholesterol, and LDL-cholesterol levels were determined by a colorimetric technique using AU 5800 automated analyzer (Beckman Coulter, CITY, STATE, USA). Thyroid-stimulating hormone (TSH), 25-hydroxyvitamin D3, and insulin levels were measured on the same device using the chemiluminescence immunoassay (CLIA) method. HbA1c levels were analyzed by high-performance liquid chromatography (HPLC) method on a Variant II turbo analyzer (Biorad, Dubai, United Arab Emirates). LDL-cholesterol levels were gained using the Friedewald formula [LDL-cholesterol = total cholesterol - (HDL-cholesterol) - (triglyceride/5)].
3.4. Statistical and Power Analysis
Before starting the study, power analysis of our study was performed with PS Power and Sample Size Calculations Version 3.0 program. When the probability of Type 1 error was taken as 0.05 in the analysis performed when each of the obese and control groups consisted of 20 patients, the probability of rejection of the hypothesis that the group means were equal was found to be 95.1% (power). Our study met these conditions with 56 obese and 32 controls.
Statistical analysis was done using statistical package for the social sciences (SPSS), version 22 (IBM Inc., Armonk, NY, USA). Normal distribution of data sets was calculated with the Shapiro-Wilk test. Descriptive statistical methods (mean, standard deviation, frequency) were used to describe data. Differences between normally distributed data sets were assessed using the one-way analysis of variance (ANOVA) test. To evaluate more than two normally distributed data sets, the Tukey’s honestly significant difference (HDS) test and the Tamhane’s T2 test were used to identify the group that was distributed significantly different. For non-parametric data sets, the Kruskal-Wallis test was utilized, and Dunn's test was used to determine the group that caused the difference. The Student’s t-test was used to compare the parameters with normal distribution, and Mann-Whitney U test was used to compare parameters with non-normal distribution between the two groups. Chi-square test and Yates’ correction were used to compare qualitative data. Pearson’s correlation analysis was used to examine the relationships between normally distributed parameters; significance was set at P < 0.05. Linear regression analysis was applied for multivariate analysis at a significance level of P < 0.05.
3.5. Ethical Considerations
The study was confirmed by the Ethics Committee of Okmeydani Training and Research Hospital, Turkey (date: 28.8.2018 and numeral: 954).
4. Results
A total of 88 age- and gender-matched adolescents (56 obese and 32 normal-weight) were enrolled in this research. The mean age of participants was 12.72 ± 1.91 years. The mean BMI was 30.92 ± 3.81 kg/m2 in obese adolescents and 23.19 ± 3.1 kg/m2 in healthy controls. There were 28 adolescents from the obese group with NAFLD on US examination and 28 obese adolescents without NAFLD.
Laboratory results for the obese and control groups are presented in Table 1. There was no statistically significant difference between the obese and control groups in terms of gender distribution, age, glucose, urea, creatinine, CRP, TSH, and 25-hydroxyvitamin D3 levels. The BMI, HbA1c, AST, ALT, total cholesterol, triglyceride, LDL-cholesterol, HOMA-IR, and insulin levels of the obese group were significantly elevated compared to the control group (P < 0.05). In contrast, omentin-1 and HDL-cholesterol levels were remarkably lower in the obese group compared to controls (P < 0.05).
| Variables | Obese Adolescents (n = 56) | Control Group (n = 32) | P |
|---|---|---|---|
| Gender; No. (%) | 0.257 b | ||
| Female | 26 (46) | 14 (43) | |
| Male | 30 (54) | 18 (57) | |
| Age (y) | 12.67 ± 1.99 | 12.81 ± 1.77 | 0.765 c |
| BMI (kg/m2) | 30.92 ± 3.81 | 23.19 ± 3.1 | < 0.0001 c, d |
| Omentin-1 (ng/mL) | 5.06 ± 4.19 | 9.3 ± 2.17 | < 0.0001 c, d |
| HbA1c (%) | 5.48 ± 0.35 | 5.22 ± 0.3 | 0.002 c, d |
| Glucose (mg/dL) | 90.42 ± 9.44 | 88.81 ± 8.77 | 0.479 c |
| Urea (mg/dL) | 24.62 ± 6.02 | 23.81 ± 6.31 | 0.591 c |
| Creatinine (mg/dL) | 0.53 ± 0.1 | 0.54 ± 0.12 | 0.923 c |
| AST (U/L) (median) | 24.53 ± 8.92 (22) | 21.08 ± 5.53 (19) | 0.035 d, e |
| ALT (U/L) (median) | 24.82 ± 11.05 (22) | 16.42 ± 7.8 (13) | < 0.0001 d, e |
| CRP (mg/L) | 6.32 ± 3.92 (6.2) | 5.12 ± 3.56 (4.2) | 0.183 e |
| Total cholesterol (mg/dL) | 168.47 ± 37.69 | 148.65 ± 28.14 | 0.023 c, d |
| Triglyceride (mg/dL) | 126.89 ± 57.51 | 81.27 ± 26.11 | < 0.0001 c, d |
| HDL-cholesterol (mg/dL) | 43.96 ± 9.33 | 49.69 ± 11.27 | 0.024 c, d |
| LDL-cholesterol (mg/dL) | 107 ± 32.55 | 86.46 ± 22.96 | 0.006 c, d |
| TSH (mU/L) | 2.78 ± 1.08 | 2.34 ± 1.1 | 0.105 c |
| 25-hydroxyvitamin D3 (ug/L) | 19.43 ± 6.21 | 17.32 ± 6.73 | 0.186 c |
| HOMA-IR (median) | 4.14 ± 2.17 (3.6) | 1.93 ± 0.72 (1.72) | < 0.0001 d, e |
| Insulin (IU/L) (median) | 18.5 ± 9.06 (16.8) | 8.72 ± 2.96 (8.2) | < 0.0001 d, e |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body-mass index; CRP, C-reactive protein; HOMA-IR, homeostatic model of assessment for insulin resistance; HDL-cholesterol, high-density lipoprotein-cholesterol; LDL-cholesterol, low-density lipoprotein-cholesterol; SD, standard deviation; TSH, thyroid-stimulating hormone.
a Values are expressed as mean ± SD unless otherwise indicated.
b Continuity (Yates) Düzeltmes.
c Student t-test.
d P < 0.05.
e Mann Whitney U test.
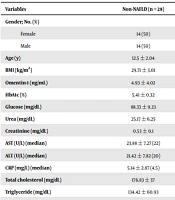
Omentin levels and other laboratory findings of the NAFLD group are compared with the non-NAFLD and control groups in Table 2. The serum omentin-1 levels of the control group were remarkably higher than the groups with or without NAFLD (P < 0.0001 and P = 0.002, respectively). There was no statistically significant difference regarding omentin-1 levels between adolescent groups with or without NAFLD (P > 0.05).
| Variables | Non-NAFLD (n = 28) | NAFLD (n = 28) | Controls (n = 32) | P |
|---|---|---|---|---|
| Gender; No. (%) | 0.318 b | |||
| Female | 14 (50) | 13 (46) | 14 (43) | |
| Male | 14 (50) | 15 (54) | 18 (57) | |
| Age (y) | 12.5 ± 2.04 | 12.86 ± 1.96 | 12.81 ± 1.77 | 0.789 c |
| BMI (kg/m2) | 29.71 ± 3.01 | 32.3 ± 4.21 | 23.19 ± 3.1 | < 0.0001 c, d |
| Omentin-1 (ng/mL) | 4.93 ± 4.02 | 5.22 ± 4.48 | 9.3 ± 2.17 | < 0.0001 c, d |
| HbA1c (%) | 5.41 ± 0.32 | 5.57 ± 0.37 | 5.22 ± 0.3 | 0.002 c, d |
| Glucose (mg/dL) | 88.33 ± 9.23 | 92.81 ± 9.31 | 88.81 ± 8.77 | 0.206 c |
| Urea (mg/dL) | 25.17 ± 6.25 | 24 ± 5.84 | 23.81 ± 6.31 | 0.709 c |
| Creatinine (mg/dL) | 0.53 ± 0.1 | 0.55 ± 0.12 | 0.54 ± 0.15 | 0.990 c |
| AST (U/L) (median) | 23.88 ± 7.27 (22) | 25.29 ± 10.64 (24) | 21.08 ± 5.53 (19) | 0.103 e |
| ALT (U/L) (median) | 21.42 ± 7.82 (20) | 28.71 ± 12.98 (26) | 16.42 ± 7.8 (13) | < 0.0001 d, e |
| CRP (mg/L) (median) | 5.14 ± 2.87 (4.5) | 7.67 ± 4.55 (6.9) | 5.12 ± 3.56 (4.2) | 0.066 e |
| Total cholesterol (mg/dL) | 176.83 ± 37 | 158.9 ± 37.02 | 148.65 ± 28.14 | 0.017 c, d |
| Triglyceride (mg/dL) | 134.42 ± 60.93 | 118.29 ± 53.49 | 81.27 ± 26.11 | 0.001 c, d |
| HDL-cholesterol (mg/dL) | 45.63 ± 9.44 | 42.05 ± 9.05 | 49.69 ± 11.27 | 0.039 c, d |
| LDL-cholesterol (mg/dL) | 111.75 ± 30.43 | 101.57 ± 34.74 | 86.46 ± 22.96 | 0.012 c, d |
| TSH (mU/L) | 2.99 ± 1.1 | 2.55 ± 1.04 | 2.34 ± 1.1 | 0.109 c |
| 25-hydroxyvitamin D3 (ug/L) | 19.64 ± 6.37 | 19.19 ± 6.16 | 17.32 ± 6.73 | 0.409 c |
| HOMA-IR (median) | 3.49 ± 1.28 (3.2) | 4.91 ± 2.72 (4.3) | 1.93 ± 0.72 (1.7) | < 0.0001 d, e |
| Insulin (IU/L) (median) | 16.11 ± 5.9 (15.2) | 21.24 ± 11.22 (19.5) | 8.7 ± 2.96 (8.3) | < 0.0001 d, e |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body-mass index; CRP, C-reactive protein; HOMA-IR, homeostatic model of assessment for insulin resistance; HDL-cholesterol, high-density lipoprotein-cholesterol; LDL-cholesterol, low-density lipoprotein-cholesterol; SD, standard deviation; TSH, thyroid-stimulating hormone.
a Values are expressed as mean ± SD unless otherwise indicated.
b Ki-Kare test.
c ANOVA.
d P < 0.05.
e Kruskal Wallis test.
The BMI values of the control group were found to be considerably lower than the adolescent groups with and without NAFLD (P < 0.0001). The BMI values of the non-NAFLD group were significantly lower than the NAFLD group (P = 0.037).
Triglyceride, HOMA-IR, and insulin levels of the control group were found to be considerably lower than the adolescent groups with and without NAFLD (P < 0.0001). There was no statistically significant difference between adolescents with or without NAFLD in terms of HOMA-IR and insulin values (P > 0.05).
HbA1c and HDL-cholesterol values of the control group were found to be remarkably lower than the obese group with NAFLD (P = 0.002).
The ALT values of the control group were found to be significantly lower than the adolescent groups with and without NAFLD (P = 0.018 and P < 0.0001, respectively). ALT values of the obese group without NAFLD were significantly lower than the obese group with NAFLD (P = 0.046). Total cholesterol and LDL-cholesterol values of the control group were considerably lower than the non-NAFLD group (P = 0.013).
Correlations between omentin-1 and other laboratory findings in the obese adolescents are shown in Table 3. In the obese adolescent group, there was a strong positive correlation between omentin-1 and ALT, and positive correlations between omentin-1 and HOMA-IR and insulin levels (P < 0.05).
| Variables | Omentin-1 | |
|---|---|---|
| r | P | |
| Age | 0.059 | 0.700 |
| BMI | 0.294 | 0.055 |
| HbA1c | 0.128 | 0.403 |
| Glucose | -0.013 | 0.935 |
| Urea | 0.143 | 0.349 |
| Creatinine | -0.090 | 0.554 |
| AST | 0.276 | 0.067 |
| ALT | 0.406 | 0.006 b |
| CRP | 0.083 | 0.588 |
| Total cholesterol | -0.077 | 0.614 |
| Triglyceride | 0.058 | 0.704 |
| HDL-cholesterol | 0.078 | 0.612 |
| LDL-cholesterol | 0.058 | 0.707 |
| TSH | -0.26 | 0.084 |
| 25-hydroxy-vitamin D3 | -0.228 | 0.132 |
| HOMA-IR | 0.304 | 0.042 b |
| Insulin | 0.300 | 0.046 b |
a Pearson correlation analysis.
b P < 0.05
When we evaluated the effects of ALT, HOMA-IR, and insulin parameters on omentin-1 values by using step-wise regression analysis, the model was found to be statistically significant (P = 0.006), and the R2 value was 0.165 (Table 4). The effect of the ALT parameter in the model was found to be statistically significant (P = 0.006). According to the model, one unit increase in ALT parameter had a small but significant 0.154-fold enhancing effect on omentin. The effect of HOMA-IR and insulin parameters on omentin-1 was not statistically significant (P > 0.05).
| Omentin-1 | B | S.E. | Beta | t | P | 95% CI | |
|---|---|---|---|---|---|---|---|
| Lowerbound | Upperbound | ||||||
| (Constant) | 1.242 | 1.433 | 0.866 | 0.391 | -1.649 | 4.132 | |
| ALT | 0.154 | 0.053 | 0.406 | 2.914 | 0.006 a | 0.047 | 0.261 |
a P < 0.05.
Correlation between omentin-1 and other laboratory values in obese groups with and without NAFLD are shown in Table 5. In the non-NAFLD group, no significant relationship was identified between omentin-1 and any parameter (P > 0.05). However, in the NAFLD group, strong positive correlations were seen between omentin-1 and BMI, AST, ALT, HOMA-IR, and insulin values (P < 0.05).
| Variables | Omentin-1 | |||
|---|---|---|---|---|
| Non-NAFLD (n = 28) | NAFLD (n = 28) | |||
| r | P | r | P | |
| Age | -0.199 | 0.352 | 0.331 | 0.143 |
| BMI | -0.034 | 0.873 | 0.550 | 0.010 b |
| HbA1c | 0.043 | 0.842 | 0.195 | 0.397 |
| Glucose | -0.018 | 0.934 | -0.025 | 0.914 |
| Urea | -0.011 | 0.961 | 0.323 | 0.154 |
| Creatinine | -0.166 | 0.438 | -0.029 | 0.902 |
| AST | -0.032 | 0.882 | 0.493 | 0.023 b |
| ALT | 0.319 | 0.129 | 0.495 | 0.024 b |
| CRP | 0.066 | 0.758 | 0.084 | 0.718 |
| Total cholesterol | -0.261 | 0.218 | 0.124 | 0.591 |
| Triglyceride | -0.241 | 0.256 | 0.423 | 0.056 |
| HDL-cholesterol | 0.087 | 0.686 | 0.086 | 0.711 |
| LDL-cholesterol | 0.029 | 0.893 | 0.096 | 0.68 |
| TSH | -0.373 | 0.072 | -0.136 | 0.555 |
| 25-hydroxy-vitamin D3 | -0.184 | 0.390 | -0.274 | 0.229 |
| HOMA-IR | 0.011 | 0.958 | 0.476 | 0.029 b |
| Insulin | -0.002 | 0.991 | 0.483 | 0.027 b |
a Pearson correlation analysis.
b P < 0.05.
The effects of BMI, AST, ALT, HOMA-IR, and insulin parameters on omentin-1 values were evaluated in the NAFLD group with step-wise regression analysis. The model was found statistically significant (P = 0.005), and the R2 value was 0.442 (Table 6). The effects of BMI and ALT parameters in the model were found to be statistically significant (P = 0.021 and P = 0.048, respectively). A one-unit increase in BMI and ALT parameters, respectively, had an enhancing effect of 0.488 and 0.133 times on omentin. The effect of AST, HOMA-IR, and insulin parameters on omentin was not statistically significant (P > 0.05).
5. Discussion
Several previous studies showed decreased levels of omentin-1 in adult obese patients (9, 11-15). Catoi et al. showed that omentin-1 levels decreased in morbidly-obese patients compared to normal-weight ones. However, they could not find any correlation among BMI, HOMA-IR, insulin or lipid panel, and omentin-1 levels (11). Auguet et al. reported that there was a connection between decreased circulating omentin-1 levels and metabolic syndrome in morbidly-obese women (12). They also reported an inverse correlation between omentin-1 levels and glucose and HOMA-IR. However, they did not identify any correlation between omentin-1 levels and BMI or insulin levels. Cimen et al. reported that omentin-1 levels decreased in an obese population and were inversely associated with BMI, insulin, and HOMA-IR, and directly associated with HDL-cholesterol (13). Ouerghi et al. stated that basal omentin-1 levels decreased in obese patients, and they also reported an increase in omentin-1 levels with decreased BMI after exercise (14).
There are also studies on the relationship between omentin-1 and markers of obesity, lipid dysregulation, and insulin resistance in the pediatric and adolescent populations. Catli et al. examined omentin-1 grades in obese children and reported increased BMI, HOMA-IR, insulin, and triglyceride levels, as well as decreased omentin-1 levels in obese children (15). While they found a negative correlation between BMI, insulin, HOMA-IR, and omentin-1 levels, they did not find any correlation between glucose, triglyceride, total cholesterol, LDL-cholesterol, HDL-cholesterol, and omentin-1 levels. They suggested that omentin-1 could be used as a biomarker for metabolic dysfunction in children and adolescents. Oswiecimska et al. reported decreased omentin-1 levels in obese adolescent girls (22).
In the present study, serum grades of omentin-1 were considerably lower in obese adolescents compared to normal-weight adolescents, which is consistent with several other reports (22). Also, in line with other studies, we showed a relationship between obesity and decreased omentin-1 levels in adolescents. We found a significant positive correlation between omentin-1 and ALT, HOMA-IR, and insulin levels in obese adolescents. However, no correlation was found between omentin-1 and BMI and other parameters.
In vitro studies have determined that recombinant omentin-1 enhances insulin signal transduction by increasing protein kinase Akt/protein kinase B phosphorylation and by enhancing insulin-stimulated glucose transport in isolated human adipocytes without any intrinsic insulin-mimic activity (8, 9). Akt is a serine/threonine-protein kinase playing a significant role as a second messenger in multiple cellular functions, including glucose metabolism, cell proliferation, and apoptosis (8). Tan et al. showed that the production of omentin-1 was reduced by both insulin and glucose in cultured adipocytes. These results show that omentin-1 production responds to the effect of both glucose and insulin (23). In our study, we found increased HbA1c, insulin, and HOMA-IR levels and decreased omentin-1 levels in the obese adolescents. It is possible that serum omentin-1 levels may be suppressed with the direct impact of increased insulin levels in obese patients.
The role of a subclinical inflammatory process has been shown in the pathogenesis of obesity (24). In obese patients, the manifestation and secretion of pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-a) and IL-6 and adipokines such as leptin and resistin increase while the levels of anti-inflammatory adipokines decrease (25). In this respect, omentin-1 levels may have been decreased in obese adolescents in our study due to the effects of inflammatory cytokines and other adipokines. Unfortunately, we did not investigate cytokine and adipokine profiles in the obese and normal-weight participants in our study. Further research is required to determine if our hypothesis is valid.
Obesity is associated with some metabolic disorders, including NAFLD. NAFLD is the most common liver disease. The term NAFLD is used to describe a wide range of fatty liver changes from simple steatosis to non-alcoholic steatohepatitis (NASH). In obesity, an increased amount of fatty acids coming into the liver, excessive intake of carbohydrates with diet, and increased fatty acid synthesis are the reasons of fatty liver (26). In current research, we divided obese adolescents into two groups with and without NAFLD, and no significant difference was present in omentin-1 levels between the two groups. This data makes it hard to determine a relation between NAFLD and omentin-1 levels, unlike obesity in adolescents. Moreover, insulin resistance and HOMA-IR levels were not remarkably different among obese adolescents with and without NAFLD. The similarity of omentin-1 levels together with the similarity in insulin and HOMA-IR levels in the NAFLD and non-NAFLD groups supports our hypothesis that omentin-1 levels decreased secondary to the effects of insulin. However, these findings of high insulin and concurrent low omentin-1 are inconclusive; obesity is a multifactorial condition and is also associated with adipose tissue dysfunction, the cells that express and secrete omentin-1.
Yılmaz et al. reported increased omentin-1 levels in patients with biopsy-proven NAFLD and determined that it was significantly associated with the grade of hepatocyte ballooning but not with hepatic steatosis or fibrosis. They also reported a positive correlation between CRP and omentin-1 levels (16). Kohan et al. reported that omentin-1 rs2274907 (326A/T) polymorphisms were considerably associated with NAFLD, and omentin-1 polymorphism could be a nominee for predisposition to NAFLD (27). Bekaert et al. showed that hepatic omentin-1 expression was lower in patients with NASH compared to those with simple steatosis. However, they found that omentin-1 serum levels were not different between patients with NAFLD and healthy controls (17). Montazerifar et al. revealed that omentin-1 levels were not different in NAFLD patients compared to the control group (18). Waluga et al. evaluated morbidly-obese patients and reported that there was no difference in serum omentin-1 levels and hepatic mRNA expression between patients with different grades of steatosis, hepatocyte ballooning, inflammatory activity, and fibrosis stage, and there was no significant relationship between plasma omentin-1 concentration or its liver mRNA expression and the concentration of glucose, insulin or HOMA-IR (28). Izadi et al. found that omentin-1 levels positively correlated with the NAFLD severity (29). In another study conducted by Aliasghari et al., serum omentin level was found to be high in patients with NAFLD (30). In this study, we found a correlation in the same direction between omentin-1 and BMI in obese adolescents with NAFLD. This might be due to a compensatory mechanism to maintain the oxidative-antioxidative stress balance in patients with NAFLD.
Physical activity is an operative tactic for fighting obesity and improving metabolic health (31-33). Ouerghi et al. reported that high-intensity exercise causes an increase in omentin-1 levels and a decrease in BMI. In this respect, physical exercise and dietary restrictions may cause serum omentin-1 levels to increase and may have beneficial effects on insulin resistance, obesity, and obesity-related diseases (14). However, the diet and exercise periods that could affect omentin-1 level were not determined in our study, which should be taken into account in future studies.
Our study had some limitations. First, our sample size was relatively small. Therefore, we could not classify the obese adolescents according to the severity of obesity. Second, we did not evaluate the subjects’ lifestyle factors such as exercise and diet.
5.1. Conclusions
Our results showed that omentin-1 levels reduced in obese adolescents. This might be due to the suppressive role of increased insulin levels and altered levels of inflammatory cytokines. Our findings make it difficult to establish a relationship between omentin-1 and NAFLD. However, this is the first study examining the relationship between NAFLD and omentin-1 in adolescents. Further research concerning omentin-1 and its place in the complex process of metabolic dysregulation because of obesity may help in the understanding of this challenging condition. Further studies may investigate approaches targeting omentin-1 in the treatment of insulin resistance diseases and obesity and its complications.