1. Background
Intussusception is very common in neonates and young children (1, 2). Conservative treatment with air or liquid contrast enema guided by fluoroscopy or ultrasound has become the first-line treatment of intussusception in children (3, 4). Surgical management is primarily applicable for those cases in which conservative management fails or those who present with intestinal necrosis or peritonitis (5-7). However, there is a lack of a good prediction model to determine whether patients should be treated by surgery in time or enema and whether enema will be successful or not.
Güney et al. pointed out that intussusception patients with peritoneal irritation should be treated by surgery (6). He et al. found that the initial location of intussusception, the presence of peritoneal effusion, the interception of intussusception, and blood in the stool might be related to the failure of conservative treatment (8). Huang et al. showed that symptom duration and hematochezia were associated with conservative treatment failure and intestinal necrosis (5). Due to the risks of radiation, children should also avoid radiation computed tomography scans and repeated X-rays. In addition, the unclear medical history of the children and the lack of cooperation in physical examination made it impossible to make a judgment on the occurrence of intestinal necrosis in children with intussusception without operation.
2. Objectives
This study aimed to screen out the main risk factors for the surgical treatment of intestinal obstruction and intestinal necrosis in children through univariate analysis and logistic regression analysis by taking the simple, measurable blood biochemical index as a factor and establish a surgical prediction model to provide help for clinical decision-making.
3. Methods
3.1. Patients
This study was performed on 8235 children with intussusception hospitalized in Jiangxi Children’s Hospital within January 2010 to January 2020. All the patients were divided into two groups, namely the group with successful hydrostatic enema treatment (the HE group, n = 5743) and the group with surgical management after unsuccessful enema treatment (the SM group, n = 2492). The SM group was divided into the managed surgically without necrosis group (the SN- group, n = 2094) and the managed surgically with necrosis group (the SN+ group, n = 398).
3.2. Conservative Management with Hydrostatic Enema
Under the ultrasound guidance, a catheter was inserted into the anus and filled with 20 - 30 mL of water into the water balloon to prevent the catheter from falling off or leaking. Water injection from the catheter should not exceed 10, 12, and 14 kPa for those younger than 1, younger than 2, and older than 2 years, respectively. Fluid imaging was performed under ultrasound, and the intubation was reset by liquid pressure. If the fluid entered the small intestine through the ileocecal part, the ileocecal valve swinging could be observed, indicating successful treatment.
3.3. Surgical Management
Surgery was performed for patients suspected of intestinal necrosis or peritonitis or/and failure of enema treatment. If there was intestinal necrosis, the necrotic segment was removed.
3.4. Laboratory and Clinical Data
All subjects’ laboratory, demographic, and clinical data were obtained by reviewing the patients’ electronic medical records. The main demographic variables were gender and age. Laboratory and clinical data mainly included routine blood tests, liver, and kidney function tests, and coagulation function tests. All variable parameters were detected at the first admission of patients.
3.5. Statistical Analysis
All patients’ data were imported into SPSS statistical software (version 26.0; SPSS Inc., Chicago, IL, US). An independent sample t-test was performed on the data conforming to the normal distribution. The Wilcoxon rank-sum test was performed on the data not conforming to the normal distribution. A P-value less than 0.05 was considered statistically significant. Substances with significant differences were selected as variables for binary logistic regression analysis, and stepwise regression analysis was performed using the forward maximum likelihood estimation (Wald) method. The variables with a significant contribution rate to the model were obtained through binary logistic regression analysis for prediction classification. The combination of substances with the best prediction performance was screened out. The receiver operating characteristic curve (ROC) evaluated the prediction ability of the model and its corresponding sensitivity and specificity.
4. Results
4.1. Analysis of Clinical Indices of HE and SM Groups
This study analyzed 74 variables in the HE and SM groups using univariate statistical analysis. With a P-value less than 0.05 as the standard, there were 55 variables with significant differences between the two groups (Table 1). The contents of 18 variables in the SM group increased, and the contents of 37 variables decreased, compared to those of the HE group. The ROC was used to evaluate the ability of univariate to diagnose and predict HE/SM. It was shown that univariate could not distinguish HE/SM well [the area under the receiver operating characteristic curve (AUROC) < 0.7)].
| Factors | HE | SM | P d | FC | AUROC | 95% CI |
|---|---|---|---|---|---|---|
| Number | 5743 | 2494 | / | / | / | / |
| Males/Females | 3986/1757 | 1690/802 | / | / | / | / |
| Age (y) | 1.54 ± 0.02 | 1.17 ± 0.04 | *** | 0.76 | 0.55 | 0.48 - 0.61 |
| CRP (mg/L) | 9.69 ± 0.19 | 14.64 ± 0.45 | *** | 1.51 | 0.56 | 0.54 - 0.58 |
| SAA (mg/L) | 72.44 ± 1.71 | 90.61 ± 3.45 | *** | 1.25 | 0.56 | 0.54 - 0.58 |
| DBIL (μmol/L) | 2.66 ± 0.02 | 3.17 ± 0.04 | *** | 1.19 | 0.57 | 0.55 - 0.60 |
| GGT (U/L) | 12.51 ± 0.14 | 14.57 ± 0.27 | *** | 1.16 | 0.61 | 0.59 - 0.63 |
| MONO# (× 109/L) | 0.80 ± 0.01 | 0.93 ± 0.01 | *** | 1.16 | 0.56 | 0.54 - 0.58 |
| TBIL (μmol/L) | 7.44 ± 0.05 | 8.61 ± 0.22 | *** | 1.16 | 0.55 | 0.53 - 0.57 |
| Glu (mmol/L) | 4.56 ± 0.04 | 5.22 ± 0.11 | *** | 1.14 | 0.62 | 0.58 - 0.66 |
| IDBL (μmol/L) | 4.78 ± 0.03 | 5.45 ± 0.19 | * | 1.14 | 0.53 | 0.50 - 0.55 |
| MONO% (%) | 7.24 ± 0.05 | 8.23 ± 0.09 | *** | 1.14 | 0.55 | 0.53 - 0.57 |
| TG (mmol/L) | 0.76 ± 0.01 | 0.86 ± 0.02 | *** | 1.13 | 0.57 | 0.53 - 0.60 |
| PLT (× 109/L) | 358.64 ± 1.51 | 404.60 ± 2.70 | *** | 1.13 | 0.61 | 0.59 - 0.62 |
| UA (µmol/L) | 314.96 ± 1.44 | 353.71 ± 2.87 | *** | 1.12 | 0.60 | 0.56 - 0.63 |
| Mb (µg/L) | 23.51 ± 0.45 | 26.39 ± 1.21 | * | 1.12 | 0.53 | 0.51 - 0.56 |
| PCT (%) | 0.38 ± 0.01 | 0.40 ± 0.00 | *** | 1.05 | 0.60 | 0.58 - 0.62 |
| NEUT# (× 109/L) | 6.78 ± 0.04 | 7.13 ± 0.08 | ** | 1.05 | 0.53 | 0.51 - 0.55 |
| WBC (× 109/L) | 11.46 ± 0.05 | 11.80 ± 0.09 | ** | 1.03 | 0.52 | 0.50 - 0.54 |
| BUN (mmol/L) | 4.14 ± 0.02 | 4.21 ± 0.04 | *** | 1.02 | 0.54 | 0.51 - 0.58 |
| Temperature (°C) | 36.96 ± 0.01 | 37.20 ± 0.04 | *** | 1.01 | 0.56 | 0.49 - 0.62 |
| Na (mmol/L) | 138.99 ± 0.05 | 137.54 ± 0.08 | *** | 0.99 | 0.58 | 0.54 - 0.62 |
| Cl (mmol/L) | 104.57 ± 0.05 | 102.59 ± 0.10 | *** | 0.98 | 0.62 | 0.58 - 0.65 |
| K (mmol/L) | 4.57 ± 0.01 | 4.48 ± 0.01 | *** | 0.98 | 0.60 | 0.56 - 0.64 |
| MPV (fL) | 10.02 ± 0.01 | 9.81 ± 0.02 | *** | 0.98 | 0.56 | 0.54 - 0.57 |
| PDW (fL) | 11.13 ± 0.02 | 10.81 ± 0.04 | *** | 0.97 | 0.55 | 0.53 - 0.57 |
| Ca (mmol/L) | 2.42 ± 0.00 | 2.35 ± 0.00 | *** | 0.97 | 0.63 | 0.60 - 0.67 |
| RBC (× 1012/L) | 4.48 ± 0.01 | 4.35 ± 0.01 | *** | 0.97 | 0.58 | 0.56 - 0.60 |
| CK-MB (U/L) | 18.49 ± 0.16 | 17.91 ± 0.32 | *** | 0.97 | 0.55 | 0.53 - 0.57 |
| LYMPH% (%) | 33.83 ± 0.18 | 32.65 ± 0.28 | *** | 0.97 | 0.55 | 0.54 - 0.57 |
| HGB (g/L) | 114.73 ± 0.15 | 110.64 ± 0.25 | *** | 0.96 | 0.59 | 0.57 - 0.61 |
| HCT (%) | 34.71 ± 0.04 | 33.43 ± 0.07 | *** | 0.96 | 0.60 | 0.58 - 0.62 |
| Mg (mmol/L) | 0.95 ± 0.00 | 0.91 ± 0.01 | *** | 0.96 | 0.52 | 0.48 - 0.56 |
| HDL (mmol/L) | 1.16 ± 0.01 | 1.11 ± 0.02 | ** | 0.96 | 0.55 | 0.51 - 0.59 |
| AST/ALT | 2.28 ± 0.02 | 2.18 ± 0.03 | *** | 0.96 | 0.56 | 0.54 - 0.58 |
| RBP (mg/L) | 21.54 ± 0.11 | 20.58 ± 0.19 | *** | 0.96 | 0.60 | 0.58 - 0.62 |
| TC (mmol/L) | 3.92 ± 0.02 | 3.73 ± 0.05 | *** | 0.95 | 0.58 | 0.54 - 0.61 |
| GLB (g/L) | 22.45 ± 0.06 | 21.30 ± 0.09 | *** | 0.95 | 0.58 | 0.56 - 0.60 |
| 5-NT (U/L) | 5.18 ± 0.04 | 4.91 ± 0.05 | *** | 0.95 | 0.50 | 0.48 - 0.52 |
| TP (g/L) | 66.68 ± 0.08 | 63.19 ± 0.14 | *** | 0.95 | 0.67 | 0.65 - 0.69 |
| ALB (g/L) | 44.22 ± 0.05 | 41.89 ± 0.09 | *** | 0.95 | 0.66 | 0.64 - 0.68 |
| AST (U/L) | 44.35 ± 0.32 | 41.97 ± 0.45 | *** | 0.95 | 0.55 | 0.52 - 0.57 |
| β-2MG (mg/L) | 1.96 ± 0.01 | 1.85 ± 0.02 | *** | 0.94 | 0.56 | 0.54 - 0.58 |
| BUN/CR | 0.17 ± 0.00 | 0.16 ± 0.00 | *** | 0.94 | 0.50 | 0.47 - 0.54 |
| P-LCR (%) | 24.91 ± 0.10 | 23.32 ± 0.15 | *** | 0.94 | 0.56 | 0.54 - 0.57 |
| CK (U/L) | 124.32 ± 1.66 | 116.27 ± 4.37 | *** | 0.94 | 0.59 | 0.56 - 0.61 |
| PA (mg/L) | 157.46 ± 0.50 | 146.52 ± 0.77 | *** | 0.93 | 0.59 | 0.57 - 0.61 |
| P (mmol/L) | 1.69 ± 0.01 | 1.57 ± 0.02 | *** | 0.93 | 0.54 | 0.50 - 0.57 |
| LDL-C (mmol/L) | 2.09 ± 0.02 | 1.92 ± 0.03 | *** | 0.92 | 0.58 | 0.54 - 0.61 |
| CK/CK-MB | 8.03 ± 0.11 | 7.23 ± 0.16 | *** | 0.90 | 0.56 | 0.54 - 0.58 |
| C1q (mg/L) | 178.73 ± 2.13 | 159.42 ± 1.38 | *** | 0.89 | 0.64 | 0.60 - 0.68 |
| Weight (kg) | 10.38 ± 0.05 | 9.18 ± 0.09 | *** | 0.88 | 0.58 | 0.51 - 0.64 |
| ALP (U/L) | 210.05 ± 1.46 | 183.08 ± 1.51 | *** | 0.87 | 0.62 | 0.60 - 0.64 |
| BASO% (%) | 0.20 ± 0.01 | 0.14 ± 0.01 | *** | 0.70 | 0.54 | 0.52 - 0.55 |
| EO#9/L) | 0.07 ± 0.00 | 0.04 ± 0.00 | *** | 0.57 | 0.63 | 0.62 - 0.65 |
| EO% (%) | 0.63 ± 0.01 | 0.36 ± 0.02 | *** | 0.57 | 0.63 | 0.62 - 0.65 |
| BASO# (× 109/L) | 0.02 ± 0.00 | 0.01 ± 0.00 | *** | 0.50 | 0.53 | 0.52 - 0.55 |
Abbreviations: AUROC, the area under the receiver operating characteristic curve; CRP, C-reactive protein; SAA, serum amyloid A; DBIL, direct bilirubin; GGT, γ-glutamyltranspeptidase; MONO#, monocyte count; TBIL, total bilirubin; Glu, glucose; IDBL, indirect bilirubin; MONO%, mononuclear cell ratio; PLT, platelet; UA, uric acid; Mb, myoglobin; PCT, plateletcrit; NEUT#, neutro cell count; WBC, white blood cell count; BUN, urea; Na, sodium; Cl, chlorine; K, potassium; MPV, mean platelet volume; PDW, width of platelet distribution; Ca, calcium; RBC, red blood cell countp; CK-MB, creatine isoenzyme; LYMPH%, lymphocyte ratio; HGB, haemoglobin ; HCT, hematocrit; Mg, magnesium; HDL, high-density lipoprotein; RBP, retinol binding protein; TC, total cholesterol ; GLB, globulin; 5-NT, 5 '-nucleotide enzyme; TP, the total protein; ALB, albumin; AST, aspertate aminotransferase; β-2MG, β-2MG macroglobulin; BUN, urea; CR, creatinine; P-LCR, large platelet ratio; CK, creatine kinase; PA, prealbumin; P, phosphorus; LDL-C, low-density lipoprotein cholesterol; C1q, complement C1q; ALP, alkaline phospatase; BASO%, basophil ratio; EO#, eosinophil count; EO%, eosinophil ratio; BASO#, basophil count.
a Values are expressed as mean ± SE.
b Fold change (FC) was calculated by the original data directly.
c ‘/’ represented the statistical significance of a p-value greater than 0.05.
d ***, **, * indicated P < 0.001, P < 0.01, and P < 0.05, respectively.
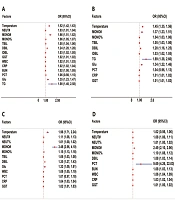
Logistic regression analysis was used to calculate the odds ratio (OR) values of different variables. It was demonstrated that the OR values of 14 variables, including γ-glutamyltranspeptidase (GGT), white blood cell count (WBC), C-reactive protein (CRP), BUN, neutro cell count (NEUT#), indirect bilirubin (IDBL), total bilirubin (TBIL), plateletcrit (PCT), mononuclear cell ratio (MONO%), direct bilirubin (DBIL), glucose (Glu), temperature, monocyte count (MONO#), and triglyceride (TG ), were greater than 1.0 (Figure 1A), suggesting that elevated levels of these 14 indicators might be associated with enema treatment failure.
Risk factors for intussusception in each group. A, risk factors for failure of hydrostatic enema therapy requiring surgical treatment; B, risk factors for failure of hydrostatic enema therapy requiring surgical treatment without intestinal necrosis; C, risk factors for failure of hydrostatic enema therapy requiring surgical treatment with intestinal necrosis; D, risk factors for immediate surgical intervention for intestinal necrosis requiring surgical treatment with intestinal necrosis.
4.2. Analysis of Clinical Indices of HE, SN-, and SN+ Groups
The SM group was further divided into SN+ and SN- groups, and the clinical indices of HE, SN-, and SN+ groups were analyzed and compared. There were 51 variables with significant differences between the HE group and SN- group compared to those of the HE group, the contents of 16 variables in the SN- group increased, and the contents of 35 variables decreased (Table 2). Each variable could not also distinguish HE/SN- (AUROC < 0.66) (Appendix 1). In the meantime, the OR value analysis results (Figure 1B) showed 11 variables, including TG, temperature, MONO#, Glu, DBIL, TBIL, MONO%, PCT, IDBL, CRP, and GGT, indicating that the elevated levels of these 11 indicators might be associated with the need for surgical treatment.
| Variables | HE | SN- | SN+ | HE vs. SN- | HE vs. SN+ | SN- vs. SN+ | |||
|---|---|---|---|---|---|---|---|---|---|
| P | FC | P | FC | P | FC | ||||
| Number | 5743 | 2094 | 398 | / | / | / | / | / | / |
| Males/Females | 3986/1757 | 1408/686 | 282/116 | / | / | / | / | / | / |
| Age (y) | 1.54 ± 0.02 | 1.18 ± 0.04 | 1.16 ± 0.11 | *** | 0.76 | *** | 0.75 | *** | 0.99 |
| Temperature (ºC) | 36.96 ± 0.01 | 37.18 ± 0.05 | 37.3 ± 0.04 | *** | 1.01 | *** | 1.01 | *** | 1.00 |
| PCT (%) | 0.38 ± 0.01 | 0.40 ± 0.01 | 0.44 ± 0.02 | *** | 1.05 | *** | 1.16 | *** | 1.10 |
| MONO% (%) | 7.24 ± 0.05 | 7.87 ± 0.09 | 10.11 ± 0.30 | *** | 1.09 | *** | 1.4 | *** | 1.28 |
| MONO# (× 109/L) | 0.80 ± 0.01 | 0.87 ± 0.01 | 1.23 ± 0.04 | *** | 1.09 | *** | 1.54 | *** | 1.41 |
| PLT (× 109/L) | 358.61 ± 1.51 | 398.09 ± 2.85 | 439.53 ± 7.53 | *** | 1.11 | *** | 1.23 | *** | 1.10 |
| GGT (U/L) | 12.52 ± 0.14 | 14.13 ± 0.28 | 16.81 ± 0.89 | *** | 1.13 | *** | 1.34 | *** | 1.19 |
| DBIL (μmol/L) | 2.66 ± 0.02 | 3.11 ± 0.04 | 3.47 ± 0.09 | *** | 1.17 | *** | 1.30 | *** | 1.12 |
| SAA (mg/L) | 72.44 ± 1.71 | 86.64 ± 3.74 | 118.44 ± 8.50 | *** | 1.20 | *** | 1.64 | *** | 1.37 |
| CRP (mg/L) | 9.69 ± 0.19 | 12.32 ± 0.36 | 26.75 ± 1.90 | *** | 1.27 | *** | 2.76 | *** | 2.17 |
| EO# (× 109/L) | 0.07 ± 0.00 | 0.04 ± 0.00 | 0.02 ± 0.00 | *** | 0.57 | *** | 0.29 | *** | 0.50 |
| EO% (%) | 0.63 ± 0.01 | 0.39 ± 0.02 | 0.17 ± 0.03 | *** | 0.62 | *** | 0.27 | *** | 0.44 |
| BASO% (%) | 0.20 ± 0.01 | 0.14 ± 0.01 | 0.12 ± 0.01 | *** | 0.70 | *** | 0.60 | * | 0.86 |
| ALP (U/L) | 210.05 ± 1.46 | 185.40 ± 1.68 | 170.81 ± 3.28 | *** | 0.88 | *** | 0.81 | *** | 0.92 |
| Weight (kg) | 10.38 ± 0.05 | 9.21 ± 0.09 | 9.04 ± 0.31 | *** | 0.89 | *** | 0.87 | *** | 0.98 |
| C1q (mg/L) | 178.73 ± 2.13 | 161.78 ± 1.48 | 142.45 ± 3.53 | *** | 0.91 | *** | 0.80 | *** | 0.88 |
| LDL-C (mmol/L) | 2.09 ± 0.02 | 1.95 ± 0.03 | 1.64 ± 0.12 | *** | 0.93 | *** | 0.78 | ** | 0.84 |
| PA (mg/L) | 157.46 ± 0.50 | 147.98 ± 0.81 | 139.01 ± 2.14 | *** | 0.94 | *** | 0.88 | *** | 0.94 |
| P (mmol/L) | 1.69 ± 0.01 | 1.60 ± 0.02 | 1.42 ± 0.04 | *** | 0.95 | *** | 0.84 | *** | 0.89 |
| ALB (g/L) | 44.22 ± 0.05 | 42.30 ± 0.09 | 39.71 ± 0.22 | *** | 0.96 | *** | 0.90 | *** | 0.94 |
| AST (U/L) | 44.35 ± 0.32 | 42.43 ± 0.47 | 39.53 ± 1.28 | *** | 0.96 | *** | 0.89 | *** | 0.93 |
| TP (g/L) | 66.68 ± 0.08 | 63.85 ± 0.15 | 59.71 ± 0.32 | *** | 0.96 | *** | 0.90 | *** | 0.94 |
| GLB (g/L) | 22.45 ± 0.06 | 21.54 ± 0.11 | 20.00 ± 0.20 | *** | 0.96 | *** | 0.89 | *** | 0.93 |
| TC (mmol/L) | 3.92 ± 0.02 | 3.77 ± 0.05 | 3.42 ± 0.13 | *** | 0.96 | *** | 0.87 | * | 0.91 |
| HCT (%) | 34.71 ± 0.04 | 33.59 ± 0.08 | 32.59 ± 0.19 | *** | 0.97 | *** | 0.94 | *** | 0.97 |
| HGB (g/L) | 114.72 ± 0.15 | 111.09 ± 0.26 | 108.32 ± 0.68 | *** | 0.97 | *** | 0.94 | *** | 0.98 |
| Mg (mmol/L) | 0.95 ± 0.00 | 0.92 ± 0.01 | 0.86 ± 0.02 | *** | 0.97 | *** | 0.91 | ** | 0.93 |
| AST/ALT | 2.28 ± 0.02 | 2.21 ± 0.03 | 2.01 ± 0.05 | *** | 0.97 | *** | 0.88 | *** | 0.91 |
| β-2MG (mg/L) | 1.96 ± 0.01 | 1.90 ± 0.03 | 1.67 ± 0.06 | *** | 0.97 | *** | 0.85 | *** | 0.88 |
| RBP (mg/L) | 21.54 ± 0.11 | 20.94 ± 0.20 | 18.80 ± 0.50 | *** | 0.97 | *** | 0.87 | *** | 0.90 |
| CK-MB (U/L) | 18.48 ± 0.16 | 17.98 ± 0.30 | 17.59 ± 1.22 | * | 0.97 | *** | 0.95 | *** | 0.98 |
| Ca (mmol/L) | 2.42 ± 0.00 | 2.36 ± 0.00 | 2.28 ± 0.01 | *** | 0.98 | *** | 0.94 | *** | 0.97 |
| RBC (× 1012/L) | 4.48 ± 0.01 | 4.38 ± 0.01 | 4.24 ± 0.03 | *** | 0.98 | *** | 0.95 | *** | 0.97 |
| K (mmol/L) | 4.57 ± 0.01 | 4.49 ± 0.01 | 4.41 ± 0.04 | *** | 0.98 | *** | 0.96 | * | 0.98 |
| Cl (mmol/L) | 104.56 ± 0.05 | 102.86 ± 0.10 | 101.19 ± 0.27 | *** | 0.98 | *** | 0.97 | *** | 0.98 |
| Na (mmol/L) | 138.99 ± 0.05 | 137.86 ± 0.09 | 135.88 ± 0.23 | *** | 0.99 | *** | 0.98 | *** | 0.99 |
| CK (U/L) | 124.31 ± 1.66 | 113.98 ± 3.73 | 128.57 ± 19.22 | *** | 0.92 | *** | 1.03 | *** | 1.13 |
| UA (µmol/L) | 314.94 ± 1.44 | 350.90 ± 3.03 | 368.76 ± 8.29 | *** | 1.11 | *** | 1.17 | / | / |
| TBIL (μmol/L) | 7.44 ± 0.05 | 8.58 ± 0.25 | 8.80 ± 0.41 | *** | 1.15 | *** | 1.18 | / | / |
| Glu (mmol/L) | 4.56 ± 0.04 | 5.24 ± 0.12 | 5.13 ± 0.19 | *** | 1.15 | ** | 1.13 | / | / |
| BASO# (× 109/L) | 0.13 ± 0.01 | 0.10 ± 0.02 | 0.05 ± 0.03 | *** | 0.77 | *** | 0.38 | / | / |
| CK/CK-MB | 8.03 ± 0.11 | 7.24 ± 0.18 | 7.19 ± 0.42 | *** | 0.90 | *** | 0.90 | / | / |
| 5-NT (U/L) | 5.18 ± 0.04 | 4.93 ± 0.06 | 4.84 ± 0.13 | *** | 0.95 | ** | 0.93 | / | / |
| P-LCR (%) | 24.91 ± 0.10 | 23.30 ± 0.16 | 23.42 ± 0.38 | *** | 0.94 | *** | 0.94 | / | / |
| PDW (fL) | 11.13 ± 0.02 | 10.79 ± 0.04 | 10.89 ± 0.10 | *** | 0.97 | *** | 0.98 | / | / |
| MPV (fL) | 10.02 ± 0.01 | 9.81 ± 0.02 | 9.82 ± 0.05 | *** | 0.98 | *** | 0.98 | / | / |
| BUN (mmol/L) | 4.14 ± 0.02 | 4.14 ± 0.04 | 4.57 ± 0.14 | *** | 1.00 | / | / | * | 1.10 |
| BUN/CR | 1.03 ± 0.03 | 1.13 ± 0.06 | 1.14 ± 0.13 | *** | 1.10 | / | / | / | / |
| TG (mmol/L) | 0.76 ± 0.01 | 0.86 ± 0.03 | 0.86 ± 0.06 | *** | 1.13 | / | / | / | / |
| HDL (mmol/L) | 1.16 ± 0.01 | 1.10 ± 0.02 | 1.15 ± 0.07 | ** | 0.95 | / | / | / | / |
| IDBL (μmol/L) | 4.78 ± 0.03 | 5.47 ± 0.21 | 5.33 ± 0.35 | ** | 1.14 | / | / | / | / |
| Mb (µg/L) | 23.51 ± 0.45 | 25.30 ± 1.08 | 32.25 ± 5.01 | / | / | *** | 1.37 | *** | 1.27 |
| NEUT# (× 109/L) | 6.78 ± 0.04 | 6.92 ± 0.08 | 8.24 ± 0.24 | / | / | *** | 1.22 | *** | 1.19 |
| WBC (× 109/L) | 11.46 ± 0.05 | 11.59 ± 0.09 | 12.93 ± 0.27 | / | / | *** | 1.13 | *** | 1.12 |
| NEUT% (%) | 58.16 ± 0.21 | 58.35 ± 0.35 | 60.86 ± 0.82 | / | / | *** | 1.05 | ** | 1.04 |
| MCHC (g/L) | 331.76 ± 0.73 | 331.32 ± 0.64 | 332.58 ± 0.72 | / | / | ** | 1.00 | ** | 1.00 |
| LYMPH% (%) | 33.83 ± 0.18 | 33.40 ± 0.31 | 28.70 ± 0.66 | / | / | *** | 0.85 | *** | 0.86 |
| CysC (mg/L) | 0.67 ± 0.01 | 0.66 ± 0.01 | 0.61 ± 0.04 | / | / | * | 0.91 | / | / |
| LYMPH# (× 109/L) | 3.79 ± 0.03 | 3.75 ± 0.04 | 3.44 ± 0.08 | / | / | * | 0.91 | / | / |
Abbreviations: NEUT%, neutro cell ratio; MCHC, average hemoglobin concentration; CysC, cystatin C; LYMPH#, lymphocyte count.
a Values are expresssed as mean ± SE.
b Fold change (FC) was calculated by the original data directly.
c ‘/’ represented the statistical significance of a P-value greater than 0.05.
d ***, **, * indicated P < 0.001, P < 0.01, and P < 0.05, respectively.
There were 54 different indices between the HE and SN+ groups. Compared to those of the HE group, the contents of 18 variables in the SN+ group increased, and the contents of 36 variables decreased (Table 2). Among them, calcium (Ca) (AUROC = 0.80), complement C1q (C1q) (AUROC = 0.78), the total protein (TP) (AUROC = 0.78), and ALB (AUROC = 0.77) performed well in distinguishing (Appendix 2). The OR value analysis results showed (Figure 1C) that 12 variables, including MONO#, temperature, DBIL, Glu, MONO%, NEUT#, WBC, PCT, TBIL, CRP, GGT, and NEUT%, might be related to the appearance of intestinal necrosis.
There were 44 variables with significant differences between the SN- group and SN+ group Compared to those of the SN- group, the contents of 16 variables in the SN+ group increased, and the contents of 28 variables decreased (Table 2). Furthermore, each variable could not distinguish SN+/SN- (AUROC < 0.70) (Appendix 3). The OR value analysis results showed (Figure 1D) that 11 variables, including PCT, MONO#, MONO%, NEUT#, DBIL, BUN, WBC, CRP, temperature, GGT, and NEUT%, might be the risk factors for SN+ (OR > 1.0).
Further analysis of the different variables among the three groups of HE, SN-, and SN+ showed that there were a total of 37 variables with significant differences, among which 9 variables’ contents were gradually increased; however, 27 variables’ contents were gradually decreased. There were significant differences in 9 variables in HE/SN- groups and HE/SN+ groups, and there was no difference in SN-/SN+ groups, indicating that these substances might be related to the failure of enema treatment but not to intestinal necrosis. However, the contents of Myoglobin (MB), NEUT#, WBC, NEUT%, MCHC, and Lymphocyte ratio (LYMPH%) were significantly different only in HE/SN+ groups and SN-/SN+ groups, which indicated that the changes in the contents of these variables might be closely related to intestinal necrosis.
4.3. Establishment of Prediction Models for HE/SM and SN-/SN+
For the construction of an appropriate prediction model, the above-mentioned substances with significant differences mentioned above were used as variables for binary logistic regression analysis, and the forward Wald method was used for stage regression analysis. The inclusion criterion of variables was a P-value less than 0.05, and the exclusion criterion of variables was a P-value greater than 0.10.
The results showed that for HE/SM groups, seven variables, including NEUT#, PLT, ALB, β-2MG, Glu, UA, and Cl, were selected for the prediction model. The prediction equation was:
P = 1/(1 + e ^ [-(6.971 + 0.243 × NEUT# + 0.002 × PLT - 0.153 × ALB - 0.392 × β-2MG - 1.236 × Glu + 0.007 × UA - 0.096 × Cl)])
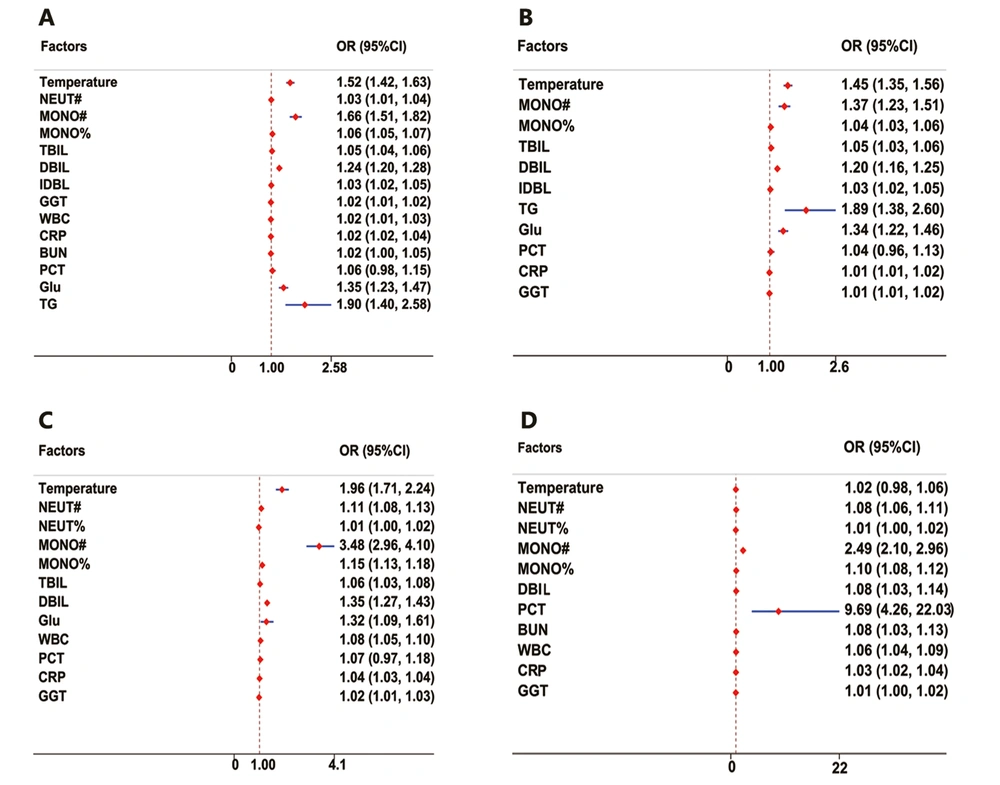
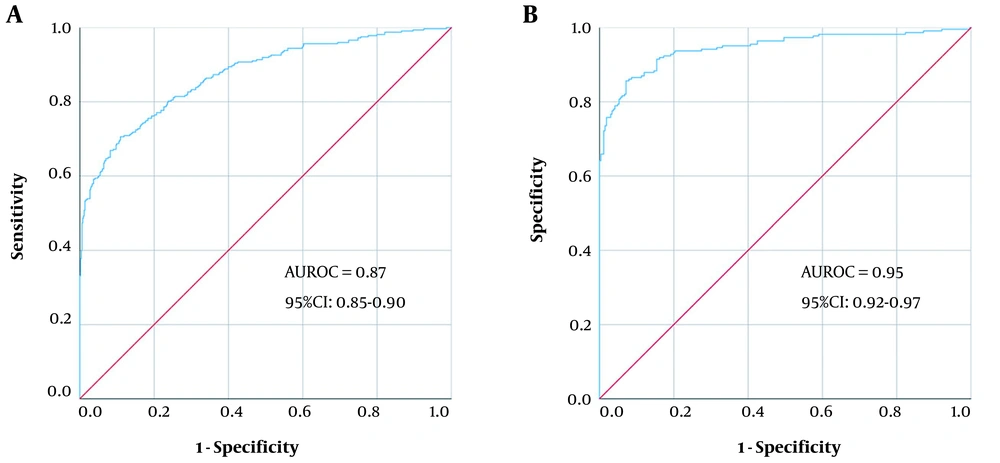
P > 0.5 was identified as SM. The model’s average predictive accuracy was 81.82%, and the AUROC was 0.87 (95% CI: 0.85 - 0.90). When the cut-off value was 0.5, the sensitivity and specificity were 70.59% and 89.08%, respectively (Figure 2A).
For HE/SM groups, six variables, including NEUT #, MCHC, PLT, TP, CRP, and BUN, were selected for the prediction model. The prediction equation was:
P = 1/(1 + e ^ [-(-50.92 + 0.328 × NEUT# + 0.175 × MCHC + 0.002 × PLT - 0.174 × TP + 0.06 × CRP + 0.279 × BUN)]
P > 0.5 was identified as SN-. The model’s average predictive accuracy was 87.93%, and the AUROC was 0.95 (95% CI: 0.92 - 0.97). When the cut-off value was 0.5, the sensitivity and specificity were 85.65% and 92.86%, respectively (Figure 2B).
4.4. Different Factors Between Patients’ Ages
Among patients younger than 1 year, compared to HE patients, the contents of 17 variables increased, and the contents of 38 variables decreased in SN- patients. Moreover, the contents of 23 variables increased, and the contents of 36 variables decreased in SN+ patients. Compared to those of SN- patients, the contents of 12 variables increased, and the contents of 28 variables decreased in SN+ patients. The contents of 33 variables showed significant differences among the HE, SN-, and SN+ groups, among which 7 variables were gradually increased; nevertheless, 25 variables were gradually decreased (Appendix 4).
Among patients aged 1 - 3 years, compared to HE patients, 14 variables’ contents increased, and 30 variables’ contents decreased in SN- patients. Furthermore, 15 variables’ contents increased, and 20 variables’ contents decreased in SN+ patients. Compared to those of SN- patients, 13 variables’ contents increased, and 13 variables’ contents decreased in SN+ patients. The contents of 16 variables showed significant differences among the HE, SN-, and SN+ groups, among which 6 variables’ contents were gradually increased; however, 9 variables’ contents were gradually decreased (Appendix 5).
Among patients aged 3 - 7 years, compared to HE patients, 7 variables’ contents increased, and 11 variables’ contents decreased in SN- patients. Additionally, 13 variables’ contents increased, and 11 variables’ contents decreased in SN+ patients. Compared to those of SN- patients, 14 variables’ contents increased, and 10 variables’ contents decreased in SN+ patients. The contents of the 5 variables showed significant differences among the HE, SN-, and SN+ groups, among which the contents of age and PLT were gradually increased; however, the content of β-2MG was gradually decreased (Appendix 6).
Among patients older than 7 years, compared to those of HE patients, 2 variables’ contents increased, and 2 variables’ contents decreased in SN- patients. Furthermore, 14 variables’ contents increased, and 7 variables’ contents decreased in SN+ patients. Compared to those of SN- patients, 14 variables’ contents increased, and 6 variables’ contents decreased in SN+ patients (Appendix 7).
By comparing the different substances in patients of all ages, it was shown that 12 substances in HE versus SN- were only discrepant in patients younger than 1 year, and 5 substances were only discrepant in patients of 1 - 3 years. Additionally, 2 substances were only discrepant in patients of 3 - 7 years, and 1 substance was only discrepant in patients older than 7 years. In HE versus SN+, 17 substances were only discrepant in patients younger than 1 year, and 1 substance was only discrepant in patients of 3 - 7 years. Moreover, 3 substances were only discrepant in patients older than 7 years. In SN- versus SN+, 11 substances were only different in patients younger than 1 year, and 2 substances were only different in patients of 1 - 3 years. Furthermore, 5 substances were only different in patients of 3 - 7 years, and 4 substances were only different in patients older than 7 years (Table 3).
| Variables | Patient Age < 1 Year | Patient Age = 1 - 3 Years | Patient Age = 3 - 7 Years | Patient Age > 7 Years | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factors | P | FC | Factors | P | FC | Factors | P | FC | Factors | P | FC | |
| HE vs. SN- | NEUT% | *** | 1.03 | IDBL | * | 1.08 | DP | *** | 1.08 | CFbg | * | 1.46 |
| LYMPH# | *** | 0.91 | AST/ALT | ** | 1.05 | Mb | * | 1.16 | ||||
| LYMPH% | *** | 0.94 | ALB/GLB | *** | 0.99 | |||||||
| WBC | ** | 0.97 | BUN | *** | 0.98 | |||||||
| RBC | *** | 0.98 | BUN/CR | *** | 0.93 | |||||||
| 5-NT | *** | 0.95 | ||||||||||
| LDH | * | 0.98 | ||||||||||
| CysC | * | 0.93 | ||||||||||
| C1q | *** | 0.93 | ||||||||||
| K | *** | 0.97 | ||||||||||
| TG | ** | 0.95 | ||||||||||
| LDL-C | ** | 0.92 | ||||||||||
| HE vs. SN+ | MONO% | *** | 1.47 | HDL | * | 1.33 | DP | ** | 1.14 | |||
| MCH | *** | 1.02 | SP | * | 1.11 | |||||||
| MCHC | *** | 1.01 | LDH | * | 0.99 | |||||||
| MCV | * | 1.01 | ||||||||||
| GGT | * | 1.21 | ||||||||||
| Glu | ** | 1.14 | ||||||||||
| BUN | * | 1.16 | ||||||||||
| CR | ** | 1.11 | ||||||||||
| BASO# | * | 0.73 | ||||||||||
| P-LCR | *** | 0.92 | ||||||||||
| MPV | *** | 0.97 | ||||||||||
| PDW | *** | 0.97 | ||||||||||
| ALT | *** | 0.83 | ||||||||||
| CysC | * | 0.88 | ||||||||||
| P | *** | 0.82 | ||||||||||
| TG | *** | 0.84 | ||||||||||
| LDL-C | *** | 0.76 | ||||||||||
| SN- vs. SN+ | Temperature | * | 1.00 | BUN | * | 1.12 | RDW-CV | ** | 1.04 | SP | * | 1.10 |
| MONO% | *** | 1.31 | UA | ** | 1.21 | ALT | * | 1.39 | MCHC | * | 1.04 | |
| Weight | ** | 0.96 | HDL | * | 1.40 | LDH | ** | 1.33 | ||||
| PA | *** | 0.93 | DP | * | 0.91 | LYMPH# | ** | 0.77 | ||||
| AST | *** | 0.93 | CK/CK-MB | * | 0.60 | |||||||
| ALP | *** | 0.93 | ||||||||||
| C1q | *** | 0.89 | ||||||||||
| Ca | *** | 0.96 | ||||||||||
| Mg | * | 0.94 | ||||||||||
| P | *** | 0.89 | ||||||||||
| TG | ** | 0.88 | ||||||||||
Abbreviations: NEUT%, neutro cell ratio; WBC, white blood cell count; RBC, red blood cell count; MCH, mean corpuscular hemoglobin; MCV, mean corpuscular volume; Glu, glucose; BASO#, basophil count; CFbg, fibrinogen; LDH, lactic dehydrogenase.
By analyzing the clinical manifestations of patients of all ages, it was shown that age had a particular influence on intussusception patients. As the age of patients decreased, more different substances were affected in each group; for example, in HE/SN-, there were 55, 45, 19, and 4 different substances in < 1 year, 1 - 3 years, 3 - 7 years, and 7 > years, respectively.
5. Discussion
This study analyzed the extensive sample data of more than 8000 children with intussusception to establish prediction models suitable for HE, SM, SN-, and SN+. Moreover, this study further analyzed the indicators’ changes in different age groups of patients with intussusception. In addition, this study demonstrated that some indicators might be the risk factors for intussusception complicated with intestinal necrosis in children.
When patients with intussusception are not diagnosed and treatment on time, it further leads to intestinal necrosis and even death as the condition is delayed. The results of this study showed that in patients with intussusception of different severity, the contents of temperature, PCT, MONO%, MONO#, GGT, DBIL, and CRP in HE, SN-, and SN+ groups increased gradually. Relative to HE, the OR values of these substances in SN- and SN+ presented an increasing trend. With increasing these indicators, as the number of patients with intussusception needing surgery increased, the risk of intestinal necrosis became greater. The results showed that these indicators might be helpful in monitoring the progression of symptoms in a child presenting with intussusception. The increased level of these markers (i.e., temperature, PCT, MONO%, MONO#, GGT, DBIL, and CRP) in severe cases of intussusception could be explained by bowel ischemia and bacterial translocation.
The published predictors of intestinal necrosis include systolic blood pressure, Lactic dehydrogenase (LDH), WBC, serum lactic acid (9-12), mesentery incarceration (13), the presence of grossly bloody stool, duration of symptom (5), shock, and hemoconcentration (14). However, there are few studies related to intestinal necrosis in children with intussusception. In this study, NEUT#, NEUT%, and WBC were the risk factors for intestinal necrosis in HE/SN- and SN-/SN+ (OR > 1.0), and these indicators played an essential role in resistance to bacterial invasion and inflammatory response, as previously described (15, 16). It was speculated that the reason might be that with the prolongation of the duration of the disease and the increased degree of intestinal damage, the degree of bacterial inflammatory reaction and bacterial injury in the children’s body were aggravated.
Intussusception was mostly observed in neonates and young children (1), and age greatly impacted the incidence rate of intussusception (17). The age might be a risk factor for the recurrence of intussusception (18-20) and failure of enema in intussusception patients (21, 22). Additionally, age was related to the need for surgical treatment and intestinal necrosis (23). The findings of the present study are in line with the results of the above-mentioned studies, as shown in Table 3 and supplementary appendices 4 - 7. It was speculated that as patients got older, their intestinal functions became perfect. The body’s self-regulation function became better and better; therefore, the incidence of intussusception would decrease, and the impact of intussusception would gradually decrease.
Although this study had explored the risk factors and predictive models for intussusception and intestinal necrosis in children, it still had numerous limitations. Firstly, this study was a retrospective cohort study, and the lack of a specific type of intussusception might make the results less objective, thereby limiting the clinical practice. Secondly, this study was conducted in one institution in China, and it was uncertain whether the obtained results were of general significance.