1. Background
Ventricular septal defect (VSD) is the most common congenital heart defect worldwide (1, 2). Perimembranous VSD accounts for almost 70% of the cases (3). Due to the advances in imaging and screening of infants, the detection rate of confirmed cases of VSD has risen considerably (4). Approximately 45% of VSDs which occur in isolation are closed spontaneously (5). Surgical treatment is often recommended for patients with medium and larger defects (1). Although traditional surgical procedures have shown excellent results, they still carry risks such as complete atrioventricular block, residual shunt, post-pericardiotomy syndrome, wound infection, reoperation, aortic regurgitation, outflow tract obstruction, and even death (2, 6, 7).
Since the introduction of transcatheter VSD closure in 1988 (8), this catheter-based approach has been widely used as an alternative to open-heart surgery with acceptable mortality and morbidity, as well as promising results (9-16). Nevertheless, this technique is also associated with complications such as complete heart block, aortic insufficiency, hemolysis, and embolization of the device (1).
2. Objectives
The effect of transcatheter closure of VSD on heart remodeling after percutaneous VSD closure has not yet been fully elucidated (17). Hence, the purpose of our study is to investigate the intermediate-term effect of the catheter-based approach for perimembranous VSD closure on heart function and ventricular recovery.
3. Methods
The present study was designed as a cross-sectional evaluation of cardiac remodeling and heart function in patients under 14 years of age who had undergone percutaneous VSD closure by occluder device from 2010 to 2020 in Namazi hospital, affiliated with Shiraz University of Medical Sciences, Shiraz, Iran. Patients were selected by a computer-based random selection method from our electronic database and data were collected and recorded in questionnaire templates with the informed consent of all participants' guardians and the approval of the ethics committee of Shiraz University of Medical Sciences (code: IR.SUMS.MED.REC.1399.196).
Patients' demographic profiles, including age, sex, body weight, duration of follow-up, echocardiography, and angiographic records regarding VSD size, size of the occluder device, and complications during angiography, were collected and recorded in the questionnaires. The patients under 14 years of age with perimembranous VSD and without any other congenital heart disease were enrolled in this study. Patients with a residual shunt, QP/QS more than 1.5, any periprocedural complications, any conduction abnormalities (right bundle branch block, left bundle branch block, heart block, and left ventricular dilation), and those with more than mild valvular regurgitation were excluded from the study. All patients were followed using M-mode, 2-dimensional, flow Doppler, and tissue Doppler imaging (TDI) echocardiography methods. All the echocardiography studies were performed by the same physician with at least 20 years of experience in the field of pediatric echocardiography.
3.1. Transthoracic Echocardiography Method
Echocardiography was performed using Samsung HS70 (Samsung Electronics Co., Ltd./Samsung Medison Co., Ltd.) with 2 - 4 and 3 - 7 MHz probe, on apical four chambers, subcostal, long axis, and short axis views. In the parasternal long axis view, left ventricular dimensions in systole and diastole, interventricular septal thickness, and ejection fraction were recorded. In four chambers view, the curser was placed on mitral and tricuspid valve leaflets, and the inflow E and A velocity was measured. In four chambers view, TDI was obtained as the cursor was placed 1 cm apical to the mitral and tricuspid annuli, and pulse wave Doppler velocity was in the -20 to +20 cm/sec.
The parameters were obtained in three cycles, and the average values were used in the study. IVSDd (interventricular septum diastolic diameter), IVSDs (interventricular septum systolic diameter), LVIDd (left ventricular internal diameter in diastole), LVIDs (left ventricular internal diameter in systole), LVPWd (left ventricular posterior wall thickness in diastole), LVPWs (left ventricular posterior wall thickness in systole), LVEF, LVFS, Em (early diastolic velocity of mitral valve), Am (atrial contractility velocity of mitral), Et (early diastolic velocity of tricuspid valve), At (atrial contractility velocity of tricuspid), EaM (early diastolic velocity of lateral mitral annulus), AaM (late diastolic velocity of lateral mitral annulus), EaT (early diastolic velocity of lateral tricuspid annulus), and Aat (late diastolic velocity of lateral tricuspid annulus) were recorded. Echocardiography data were expressed as Z-scores according to previously published Z-score values in the corresponding pediatric age group (18-20).
3.2. Statistical Analysis
Descriptive data were presented as means and standard deviations (SD), frequencies, and percentages. Normal distribution of data was obtained by Kolmogorov-Smirnov, and differences in continuous variables were compared using an independent t-test. Pearson correlation was used to analyze univariate associations between continuous variables. The Mann-Whitney U test was used for nonparametric variables. All the analysis was performed using SPSS for Windows (version 22). P-value less than 0.05 was considered as statistical significance.
4. Results
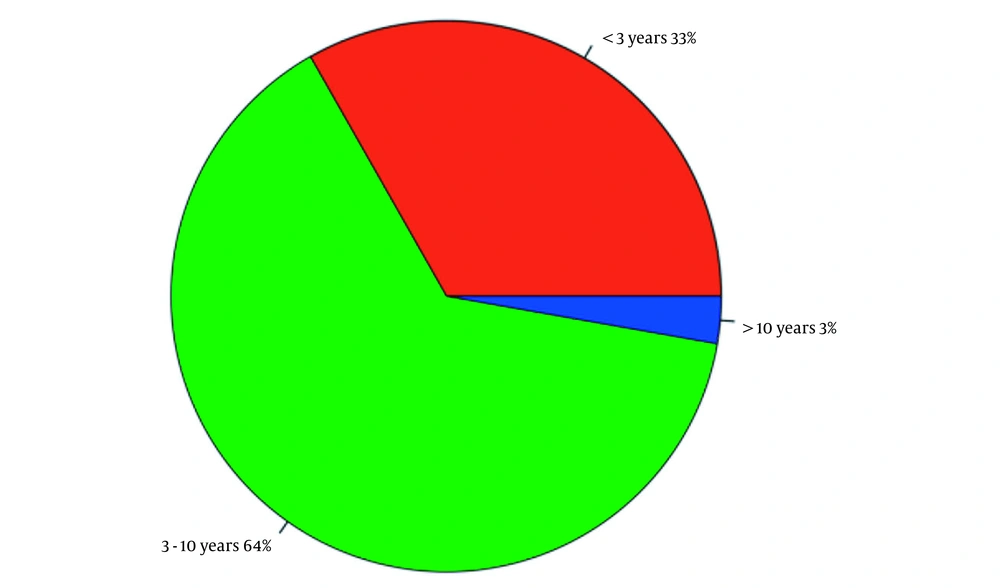
In this study, a total of 46 patients (32 males; 69.6%) with a mean age of 4.77 ± 2.69 years and mean weight of 16.27 ± 6.05 kg were randomly selected. The demographic and clinical characteristics of the participants are shown in (Table 1). As can be seen, 32.4% of patients were younger than three years of age, 62.2% of patients aged less than five years of age, while only 2.7% were older than ten years of age (Figure 1).
| Variables | Mean ± Standard Deviation | Range |
|---|---|---|
| The patients’ age at the time of catheterization (y) | 4.77 ± 2.69 | 1.40 - 13.90 |
| The patients’ weight at the time of catheterization (kg) | 16.27 ± 6.05 | 9.00 - 40.00 |
| The patients’ body surface area at the time of catheterization (m2) | 0.69 ± 0.21 | 0.43 - 1.28 |
| Size of the VSDs (mm) | 6.73 ± 2.37 | 4.00 - 14.00 |
| Size of the occluder device (mm) | 8.52 ± 2.32 | 6.00 - 16.00 |
| Duration of follow-up (mon) | 15.76 ± 12.20 | 2.00 - 48.00 |
In M-mode echocardiography, 84.6% had IVSDd Z-score ≥ 2; 23.8% had IVSDs Z-score ≥ 2; 38.5% had LVIDd Z-score ≥ 2; 34.6% had LVIDs Z-score ≥ 2; and 65.4% had LVPWd Z-score ≥ 2. Table 2 demonstrates the characteristics of M-mode, inflow Doppler, and tissue doppler echocardiography. In the evaluation of Doppler and tissue Doppler, 36.4% of the patients had Z-score ≥ 2 for E/Ea of tricuspid valve. Other parameters were within normal limits (Table 2).
| Variables | Mean ± SD | The Percentage of Patients with Z- Score ≥ 2 | The Percentage of the Patients with Z- Score ≤ -2 |
|---|---|---|---|
| M-mode echocardiographic data of left ventricle | |||
| IVSd Z-score (cm) | 3.59 ± 2.48 | 84.6 | 0 |
| IVSs Z-score (cm) | 1.44 ± 1.07 | 23.8 | 0 |
| LVIDd Z-score (cm) | 1.72 ± 1.20 | 38.5 | 0 |
| LVIDs Z-score (cm) | 1.27 ± 1.39 | 34.6 | 0 |
| LVPWd Z-score (cm) | 2.42 ± 1.59 | 65.4 | 0 |
| LVPWs Z-score (cm) | -0.57 ± 1.08 | 0 | 9.5 |
| EF% | 68.78 ± 9.69 | - | - |
| FS% | 38.56 ± 7.72 | - | - |
| Doppler and tissue Doppler data of the tricuspid and mitral valves | |||
| ET Z-score | 0.38 ± 1.14 | 6.9 | 3.4 |
| AT Z-score | 0.83 ± 1.14 | 17.9 | 0 |
| ET/AT Z-score | -0.37 ± 0.93 | 0 | 3.6 |
| EM Z-score | -0.60 ± 0.80 | 0 | 9.1 |
| AM Z-score | 0.09 ± 1.04 | 6.1 | 0 |
| EM/AM Z-score | -0.50 ± 0.77 | 0 | 0 |
| EaT Z-score | -0.78 ± 1.11 | 3.8 | 3.8 |
| AaT Z-score | 0.60 ± 1.22 | 11.5 | 0 |
| EaM Z-score | -0.87 ± 1.10 | 0 | 17.1 |
| AaM Z-score | 0.63 ± 1.17 | 7.1 | 0 |
| ET/EaT Z-score | 1.31 ± 1.43 | 36.4 | 0 |
| EM/EaM Z-score | 0.33 ± 0.89 | 9.1 | 0 |
Abbreviations: EF, ejection fraction; FS, fractional shortening.
Echocardiographic data were compared between the patients whose VSD was closed before and after three years of age. Z-scores of EaT and ET/EaT Z-score were significantly higher in patients older than three years of age than those who aged less than three years (P = 0.031). The comparison of variables is shown in Table 3.
| Variables | Comparison Group | Comparison Group | Comparison Group | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Age ≤ 3 Years (12 Patients) | Age > 3 Years (34 Patients) | P-Value | Weight ≤ 15 kg (13 Patients) | Weight > 15 kg (33 Patients) | P-Value | VSD ≤ 10 mm (9 Patients) | VSD > 10 mm (37 Patients) | P-Value | |
| IVSd Z-score | 3.80 ± 1.54 | 3.50 ± 2.84 | 0.129 | 3.55 ± 1.54 | 3.63 ± 3.14 | 0.297 | 3.32 ± 2.15 | 4.33 ± 3.31 | 0.395 |
| IVSs Z-score | 1.88 ± 0.83 | 1.27 ± 1.13 | 0.267 | 1.67 ± 0.71 | 1.24 ± 1.32 | 0.314 | 1.28 ± 0.99 | 2.99 ± 0.16 | 0.038 |
| LVIDd Z-score | 2.28 ± 1.32 | 1.47 ± 1.09 | 0.196 | 2.09 ± 1.23 | 1.41 ± 1.14 | 0.193 | 1.81 ± 1.18 | 1.50 ± 1.34 | 0.866 |
| LVIDs Z-score | 0.85 ± 1.72 | 1.45 ± 1.23 | 0.429 | 0.86 ± 1.77 | 1.62 ± 0.88 | 0.432 | 1.20 ± 1.52 | 1.46 ± 1.04 | 0.910 |
| LVPWd Z-score | 2.45 ± 1.62 | 2.41 ± 1.62 | 0.892 | 2.45 ± 1.33 | 2.40 ± 1.84 | 0.899 | 2.41 ± 1.36 | 2.45 ± 2.23 | 0.735 |
| LVPWs Z-score | -0.02 ± 0.33 | -0.80 ± 1.21 | 0.112 | -0.04 ± 1.12 | -1.06 ± 0.84 | 0.008 | -0.48 ± 1.10 | -1.46 ± 0.15 | 0.190 |
| LVEF | 73.47 ± 9.28 | 67.03 ± 9.38 | 0.055 | 73.63 ± 9.92 | 66.96 ± 9.10 | 0.037 | 69.40 ± 9.99 | 67.81 ± 9.40 | 0.673 |
| LVFS | 42.23 ± 8.00 | 37.18 ± 7.27 | 0.067 | 42.47 ± 8.56 | 37.09 ± 6.97 | 0.043 | 39.07 ± 8.10 | 37.74 ± 7.25 | 0.736 |
| EM Z-score | -0.41 ± 0.94 | -0.69 ± 0.74 | 0.585 | -0.50 ± 0.65 | -0.65 ± 0.89 | 0.927 | -0.56 ± 0.74 | -0.73 ± 1.03 | 0.726 |
| AM Z-score | -0.18 ± 1.02 | 0.22 ± 1.04 | 0.281 | -0.39 ± 0.75 | 0.37 ± 1.09 | 0.089 | 0.00 ± 0.93 | 0.39 ± 1.35 | 0.420 |
| EM/AM Z-score | -0.18 ± 0.80 | -0.65 ± 0.73 | 0.166 | -0.08 ± 0.82 | -0.73 ± 0.65 | 0.036 | -0.46 ± 0.51 | -0.61 ± 1.35 | 0.290 |
| ET Z-score | 0.09 ± 1.04 | 0.55 ± 1.20 | 0.387 | 0.41 ± 0.96 | 0.36 ± 1.26 | 0.946 | 0.54 ± 1.17 | -0.22 ± 0.88 | 0.158 |
| AT Z-score | 0.59 ± 0.80 | 0.98 ± 1.31 | 0.458 | 0.87 ± 0.72 | 1.14 ± 1.22 | 0.045 | 0.78 ± 1.16 | 0.99 ± 1.16 | 0.566 |
| ET/AT Z-score | -0.47 ± 0.73 | -0.31 ± 1.06 | 1.000 | 0.58 ± 0.23 | -0.63 ± 0.95 | 0.018 | -0.26 ± 0.86 | -0.78 ± 1.14 | 0.460 |
| EaM Z-score | -0.81 ± 1.25 | -0.91 ± 1.04 | 0.461 | -0.88 ± 0.93 | -0.87 ± 1.19 | 0.400 | -0.87 ± 1.18 | -0.88 ± .084 | 0.802 |
| EM/EaM Z-score | 0.45 ± 1.09 | 0.27 ± 0.80 | 0.611 | 0.36 ± 0.98 | 0.31 ± 0.86 | 0.868 | 0.33 ± 0.91 | 0.33 ± 0.91 | 1.000 |
| EaT Z-score | -0.95 ± 0.21 | -1.14 ± 0.53 | 0.031 | -0.28 ± 1.70 | -1.05 ± 0.52 | 0.220 | -0.96 ± 0.67 | 0.15 ± 2.41 | 0.607 |
| ET/EaT Z-score | 0.60 ± 0.77 | 1.81 ± 1.59 | 0.051 | 0.97 ± 1.41 | 1.47 ± 1.46 | 0.490 | 1.43 ± 1.40 | 0.58 ± 1.69 | 0.523 |
| AaM Z-score | 1.04 ± 1.45 | 0.42 ± 0.97 | 0.327 | 0.82 ± 1.59 | 0.54 ± 0.91 | 0.745 | 0.38 ± 0.98 | 1.51 ± 1.40 | 0.030 |
| AaT Z-score | 0.60 ± 1.84 | 0.60 ± 0.69 | 0.220 | 0.60 ± 1.36 | 0.60 ± 1.19 | 0.634 | 0.47 ± 1.08 | 1.30 ± 1.90 | 0.429 |
a Values are expressed as mean ± SD.
Patients were divided into two groups regarding their weight with a cut-off point of 15 kg. Z-scores for LVPWs, LVEF, LVFS, EM/AM, and ET/AT were higher in patients less than 15 kg at the time of VSD closure (P = 0.008, P = 0.037, P = 0.043, P = 0.036, and P = 0.018, respectively). However, AT Z-score was lower in them compared to patients weighing more than 15 kg (P = 0.045). Mean ± SDs and P-values are as shown in Table 3.
Patients were divided into two groups regarding their VSD size with a cut-off point of 10 mm. IVSDs Z-score was lower in patients with a VSD size of less than 10 mm than those with a VSD size of more than 10 mm (P = 0.038). AaM Z-score was lower in patients with VSD size of less than 10 mm compared to those with VSD size of more than 10 mm (P = 0.030). Mean ± SD and P-values are demonstrated in Table 3.
There was a positive correlation between the patients’ age and AT Z-score (P = 0.014, r = 0.458). Moreover, the patients’ weight had positive correlation with ET Z-score (P = 0.038, r = 0.426) and AT Z-score (P = 0.001, r = 0.631). VSD size of the patients had a positive correlation with IVSDs Z-score (P = 0.015, r = 0.537), while it was negatively correlated with EM Z-Score (P = 0.015, r = -0.470) and ET/EaT Z-score (P = 0.029, r = -0.499).
5. Discussion
Perimembranous VSD is the most frequent subtype of congenital heart disease (CHD) (21). Transcatheter closure of VSD has been preferred in several countries due to imposing less invasion and showing promising outcomes (22).
In the present study, we compared the patients’ echocardiographic variables with published Z-scores reported according to body surface area. A significant number of patients had an abnormally high interventricular and posterior wall thickness, and the size of VSD had a positive correlation with septal thickness. Aminullah et al. studied 24 patients with mean age of 12.60 ± 12.09 years who had undergone surgical closure of VSD. They found that left ventricular posterior wall thickness and interventricular septum thickness decreased three months after surgery, and the changes were more significant in the younger age group (23). Cordell et al. studied post-surgical VSD closure LV function and LV mass in the first two years of life, and suggested that when early surgical closure of VSD is necessary, promising results in terms of postoperative left ventricular size and function can be expected. They demonstrated that LV mass was mildly elevated at the preoperative assessment, which was decreased significantly following surgical repair (24).
In our study, left ventricular dilation was observed in about one-third of the patients. In contrast, Zheng et al. evaluated 30 patients following transcatheter closure of VSD and reported that left ventricular end-diastolic diameter and left ventricular end-diastolic volume both started to decrease three days after the intervention, and this trend continued for six months (17). Abdelrazek Ali et al. evaluated left ventricular systolic function after VSD closure using speckle tracking, which showed decreased LV volume overload with improved contractility (25).
In the evaluation of Doppler and tissue Doppler, we witnessed that one-third of the patients had Z-score of E/Ea of tricuspid more than normal, showing persistence of right-sided diastolic abnormality. In a study conducted by Klitsie et al., after one year of surgical VSD closure, LV systolic function became normal. In contrast, RV systolic function remained impaired up to 20 months after surgery (26).
Long-term evaluation of the patients after surgical perimembranous VSD closure showed long-term survival in the patients with perimembranous VSD closure, but not without any event. Some patients established significant aortic regurgitation or left ventricular outflow obstruction regardless of VSD repair. Some subjects without any predisposing factor developed atrial arrhythmia who needed pacemaker implantation (27).
In the present study, the patients' age correlated positively with AT Z-score, and their weight correlated positively with ET Z-score and AT Z-score, and negatively with EM-Z Score and ET/EaT Z-score. More studies are needed to evaluate the significance of these parameters in patients' future.
This study had some limitations. Some data were extracted retrospectively, which led to missing values and deacreased statistical power. A prospective study with a larger sample size and longer follow-up duration would provide more robust evidence about ventricular remodeling after percutaneous intervention, as well as determining the diagnostic and prognostic significance of Doppler and tissue Doppler parameters.
5.1. Conclusions
According to the results of this study, in the midterm follow-up after percutaneous closure of perimembranous VSD, left ventricular dilation and hypertrophy persisted in a significant number of patients. Early closure of VSD at lower ages and in patients with lower weights can affect the remodeling and hemodynamics of ventricles.