1. Background
In children, cancer is a leading cause of death worldwide, and each year, approximately 300,000 children aged 0-19 years are newly diagnosed with cancer (1). In childhood cancers, survival rates have increased pleasantly in the last three to four decades with intensive treatment approaches and supportive treatments. However, the survival rates vary significantly depending on the pathology, stage, and age at diagnosis (2). The cancer progression depends on the complex interaction between the tumor and the host’s inflammatory response. Although the basis of the systemic response in cancer patients is unclear, the host responds to such systemic inflammatory reaction through changes in neuroendocrine metabolism, hematopoietic changes, including interleukins, interferons, and hematopoietic growth factors, and acute phase proteins (3, 4).
Recently, many inflammatory indices or their combination, such as systemic inflammatory response index (SIRI), systemic immune-inflammation (SII) index, neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), lymphocyte-monocyte ratio (LMR), C-reactive protein-albumin ratio, and hemoglobin-albumin-lymphocyte-platelet (HALP) score have been used to predict prognosis in adults with cancer (5-9). Unlike adults, the studies on such biomarkers showing biological inflammatory status in children with cancer are limited (10-12). Tezol et al. (12) investigated these biomarkers in children with reactive lymphadenopathy and children with lymphoma. They found that NLR, LMR, PLR, and red blood cell distribution width values were significantly higher in children with lymphoma than in children with reactive lymphadenopathy.
2. Objectives
In this study, we aimed to investigate the importance of the inflammatory biomarkers showing biological inflammatory status, including SIRI, SII index, and HALP score in children with cancer.
3. Methods
In this retrospective study, between August 2018 and December 2020, oncology charts of children diagnosed with cancer in the Faculty of Medicine, Selcuk University, Division of Pediatric Hematology and Oncology, were analyzed retrospectively. The children with leukemia or retinoblastoma were not included in our study. As the control group, healthy children of matched gender and age without any known disease or infection were included.
The inclusion criteria were all newly diagnosed children with cancer who had chemotherapy in their treatment. The exclusion criteria were bone marrow involvement, patients with the finding of tumor lysis syndrome, paraneoplastic syndrome, and kidney or liver dysfunction.
Demographic and clinical characteristics of the patients were noted. International Classification of Childhood Cancer-3 was used for classification (13). The patients were divided into lymphomas, central nervous system tumors, and solid tumors groups. Since there was no staging for all childhood cancers, the patients were classified as cases with localized and advanced disease (14).
Complete blood counts, biochemical analysis results, CRP, procalcitonin, and erythrocyte sedimentation rates of the patients were recorded. The inflammatory biomarkers were calculated from complete blood count and biochemical analysis. The calculations were defined as follows: NLR: the ratio of neutrophil count to lymphocyte count, PLR: the ratio of platelet count to lymphocyte count, LMR: the ratio of lymphocyte count to monocyte count, SII index: platelet count × NLR, SIRI: [neutrophil count × monocyte count] / lymphocyte count, and HALP score: [Hemoglobin (g/L) × albumin (g/L) × lymphocyte count (/L)] / platelet count (/L) (15, 16).
3.1. Statistical Analysis
GraphPad Prism 9.0 (GraphPad, San Diego, USA) was used for all statistical analyses. As descriptive statistics, frequency and percentage values for categorical data and mean and standard deviation were used for numerical data when the distribution of the data was normal, and median values and quartile 1 and 3 (Q1 and Q3) values were given when the distribution of the data was not normal. To evaluate the distribution of numerical data, the D’Agostino & Pearson test was used upon the recommendation of GraphPad Prism. Since not meeting the necessary assumptions, the Mann-Whitney U test was used for the comparison of the two groups, and the Kruskal-Wallis test was used for the comparison of more than two groups. As a post hoc test, Dunn's multiple comparisons test was used for the Kruskal-Wallis test. If the “P” or “adjusted P” value was < 0.05, it was considered statistically significant.
4. Results
In the experimental group, there were 49 males (50.5%) and 48 females (49.5%) with a median age of 7 years (range, 3 months to 18 years). In the control group, there were 50 girls (51.5%) and 47 boys (48.5%) with a median age of 7 years (range, 3 months to 17 years). There was no statistical difference between the experimental and control groups in terms of age and gender (P values: 0.92, and 0.88, respectively).
4.1. Comparison of the Experimental and Control Groups
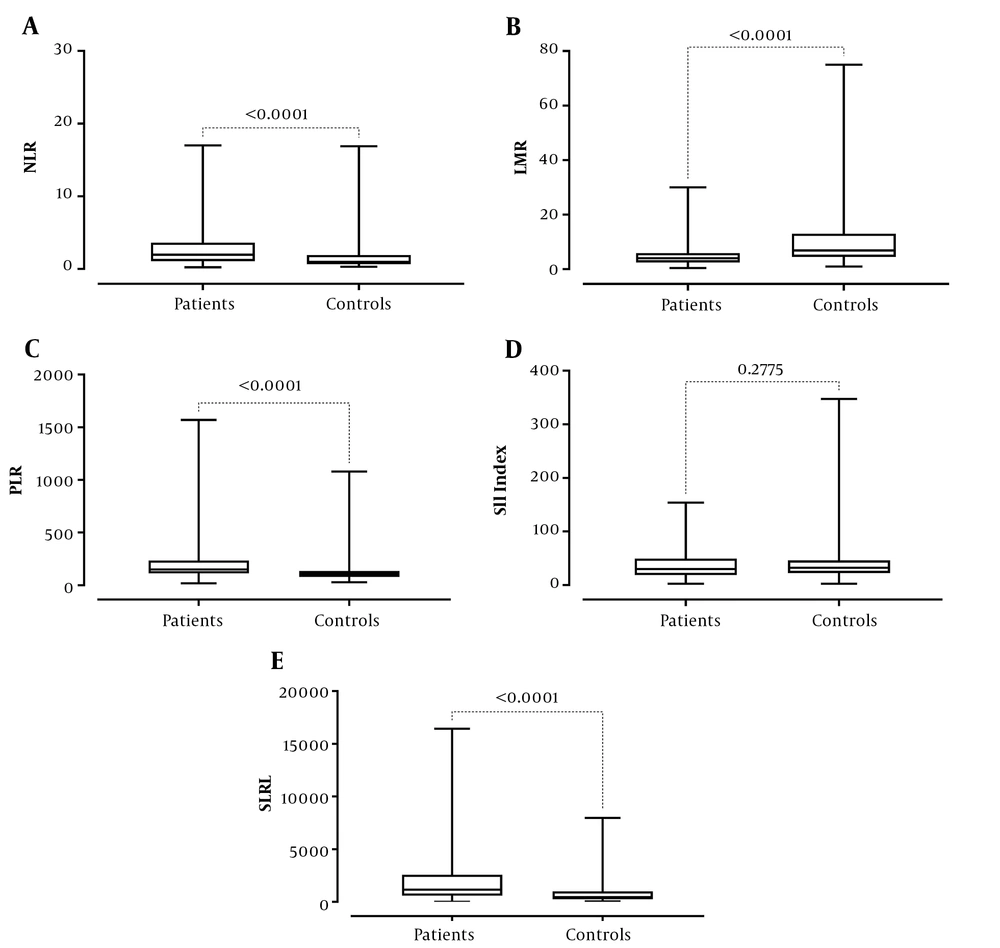
The experimental and control groups are compared in Table 1. The neutrophil, monocyte, and platelets counts, NLR and PLR ratios, and SIRI of the patients were statistically higher than the control group (P values: < 0.0001, < 0.0001, 0.0002, < 0.0001, < 0.0001, and < 0.0001, respectively) while lymphocyte and basophil counts, hemoglobin levels, and lymphocyte-to-monocyte ratio were statistically lower (P values: 0.0004, .0074, < 0.0001, and < 0.0001, respectively) (Table 1 and Figure 1). There was no statistically significant difference between leukocyte and eosinophil counts and SII index values between the experimental and the control groups (all P values > 0.05).
| Parameters | Experimental | Controls | P Value |
|---|---|---|---|
| Mdn leukocyte counts, (/µL) | 8450 | 7500 | 0.089 |
| (Q1-Q3) | (6210-11045) | (6400-9450) | |
| Mdn neutrophil counts, (/µL) | 4600 | 3230 | < 0.0001 |
| (Q1-Q3) | (3400-6775) | (2615-4590) | |
| Mdn lymphocyte counts, (/µL) | 2200 | 3100 | 0.0004 |
| (Q1-Q3) | (1700-3250) | (2190-4249) | |
| Mdn monocyte counts, (/µL) | 610 | 480 | < 0.0001 |
| (Q1-Q3) | (500-800) | (285-600) | |
| Mdn eosinophil counts, (/µL) | 100 | 150 | 0.19 |
| (Q1-Q3) | (30-200) | (83-260) | |
| Mdn basophil counts, (/µL) | 0 | 40 | 0.0074 |
| (Q1-Q3) | (0-100) | (20-75) | |
| Mdn platelets count, (/µL) | 377000 | 322000 | 0.0002 |
| (Q1-Q3) | (303000-476500) | (274000-373000) | |
| Mdn hemoglobin levels (g/dL) | 12.1 | 12.9 | < 0.0001 |
| (Q1-Q3) | (10.5-13.2) | (12.4-13.5) | |
| Mdn neutrophil-to-lymphocyte ratio | 1.96 | 0.97 | < 0.0001 |
| (Q1-Q3) | (1.085-3.625 | (0.675-1.93) | |
| Mdn lymphocyte-to-monocyte ratio | 4 | 6.87 | < 0.0001 |
| (Q1-Q3) | (2.448-5.89) | (4.505-13.02) | |
| Mdn platelet-to-lymphocyte ratio | 149.1 | 105.6 | < 0.0001 |
| (Q1-Q3) | (113.3-233.6) | (78.6-136.7) | |
| Mdn systemic immune-inflammatory index | 29.75 | 32.29 | 0.27 |
| (Q1-Q3) | (18.57-49.41) | (22.45-45.94) | |
| Mdn systemic inflammatory response index | 1150 | 442.9 | < 0.0001 |
| (Q1-Q3) | (591.2-2568) | (248.6-993.6) |
Abbreviations: Mdn, median, Q1, quartile 1; Q3, quartile 3.
4.2. Comparison of Subgroups of Children with Cancer
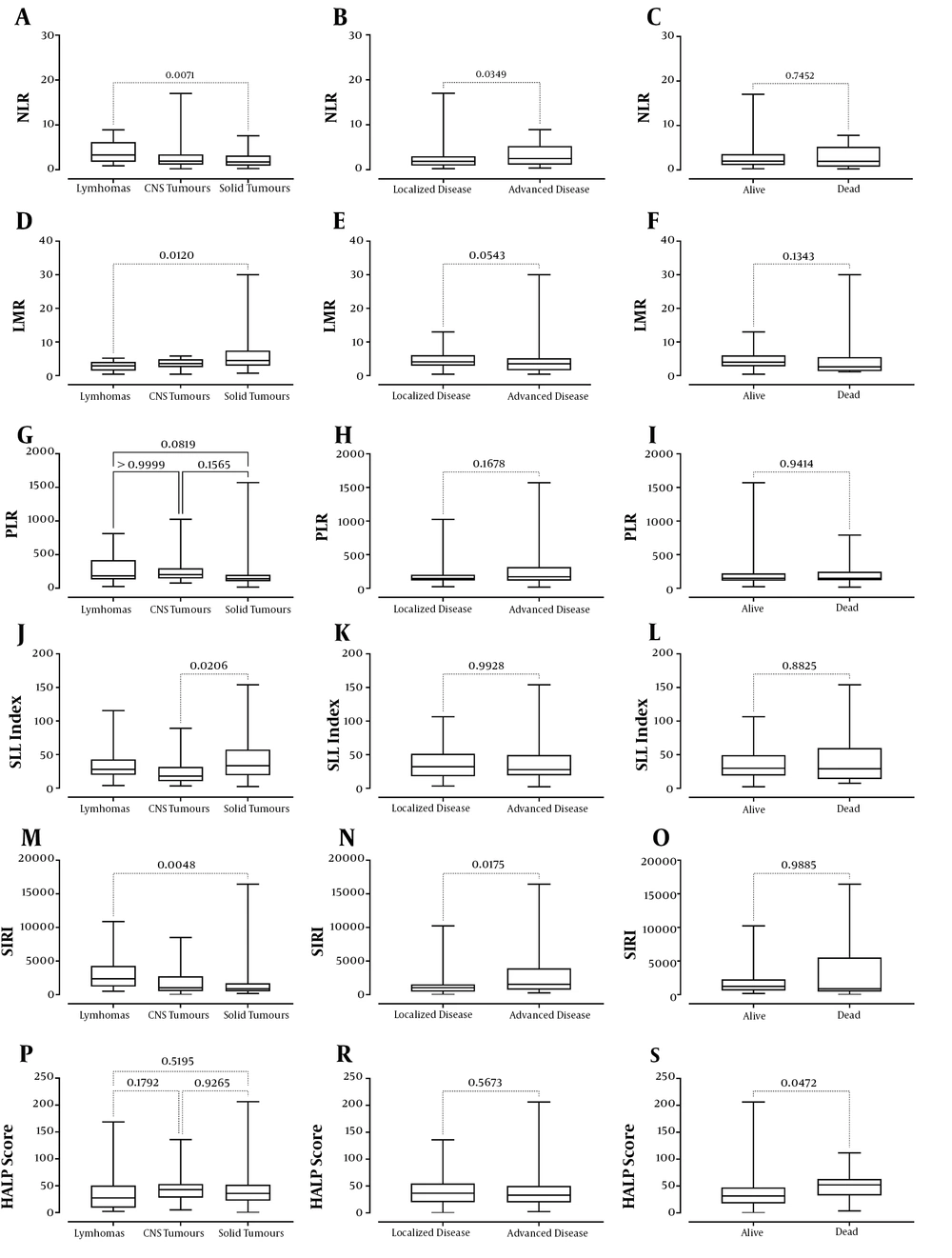
According to the diagnosis of lymphoma, tumors of the central nervous system (CNS), and solid tumors, when hematological and inflammatory biomarkers were examined, there was a statistical difference between the groups regarding the medians of lymphocyte count, NLR, LMR, PLR, SII index, SIRI, and CRP levels (P values: 0.0067, 0.0096, 0.0088, 0.0286, 0.0245, 0.0060, and 0.038, respectively) (Table 2). A significant difference was found in terms of lymphocyte count, NLR, LMR, SII index, SIRI, and CRP levels between lymphomas and solid tumors, whereas a significant difference was between CNS tumors and solid tumors regarding SII index (Table 2 and Figure 2). There was no significant difference among the groups considering the counts of leukocyte, neutrophil, monocyte, eosinophil, basophil, and platelet, hemoglobin levels, erythrocyte sedimentation rate, procalcitonin levels, and HALP score (all P values >0.05).
| Parameters | Lymphoma, (n = 20) | Tumor of the CNS, (n = 15) | Solid Tumor, (n = 62) | P Value |
|---|---|---|---|---|
| Mdn leukocyte counts, (/µL) | 8575 | 6500 | 9050 | 0.079 |
| (Q1-Q3) | (7033-10650) | (4300-9030) | (6675-11925) | |
| Mdn neutrophil counts, (/µL) | 5730 | 3600 | 4600 | 0.094 |
| (Q1-Q3) | (3833-7575) | (2380-4800) | (3343-6350) | |
| Mdn lymphocyte counts, (/µL) | 2000 | 2000 | 2550 | 0.0067a |
| (Q1-Q3) | (1083-2300) | (1000-3200) | (1875-3848) | |
| Mdn monocyte counts, (/µL) | 720 | 600 | 675 | 0.176 |
| (Q1-Q3) | (578-900) | (300-740) | (500-800) | |
| Mdn eosinophil counts, (/µL) | 100 | 100 | 100 | 0.64 |
| (Q1-Q3) | (0-290) | (0-200) | (100-205) | |
| Mdn basophil counts, (/µL) | 0 | 40 | 0 | 0.39 |
| (Q1-Q3) | (0-45) | (0-100) | (0-100) | |
| Mdn platelets count, (/µL) | 356500 | 343000 | 379500 | 0.6 |
| (Q1-Q3) | (313500-509250) | (251000-523000) | (310750-474250) | |
| Mdn hemoglobin levels, (g/dL) | 12.3 | 11.6 | 11.9 | 0.95 |
| (Q1-Q3) | (9.9-13.3) | (9.6-13.4) | (10.5-13.2) | |
| Mdn neutrophil-to-lymphocyte ratio | 3.31 | 1.95 | 1.75 | 0.0096b |
| (Q1-Q3) | (1.79-6.18) | (1.12-3.43) | (0.93-3.17) | |
| Mdn lymphocyte-to-monocyte ratio | 2.88 | 3.57 | 4.48 | 0.0088c |
| (Q1-Q3) | (1.45-4.09) | (2.51-4.88) | (2.93-7.45) | |
| Mdn platelet-to-lymphocyte ratio | 183.6 | 202 | 140.4 | 0.0286d |
| (Q1-Q3) | (131.8-417.8) | (143.8-296.5) | (104.6-199.2) | |
| Mdn systemic immune-inflammatory index | 27.83 | 17.82 | 33.45 | 0.0245e |
| (Q1-Q3) | (19.78-42.79) | (10.33-31.65) | (19.19-57.35) | |
| Mdn systemic inflammatory response index | 2363 | 1029 | 875.3 | 0.0060f |
| (Q1-Q3) | (1203-4293) | (530.8-2736) | (492.2-1690) | |
| Mdn HALP score | 27.33 | 42.81 | 35.74 | 0.15 |
| (Q1-Q3) | (9.09-50.5) | (27.89-53.34) | (22.26-52.05) | |
| Mdn C-reactive protein, (mg/dL) | 2.11 | 0.35 | 0.57 | 0.038g |
| (Q1-Q3) | (0.36-13.5) | (0.14-1.44) | (0.21-1.84) | |
| Mdn erythrocyte sedimentation rate | 19 | 19 | 22 | 0.62 |
| (Q1-Q3) | (10-54.5) | (7-45) | (7-40) | |
| Mdn, procalcitonin, (µg/L) | 0.1 | 0.1 | 0.09 | 0.42 |
| (Q1-Q3) | (0.05-0.3) | (0.05-0.38) | (0.05-0.24) |
Abbreviations: Mdn, median; Q1, quartile 1; Q3, quartile 3.
aLymphomas & solid tumors, adjusted P value = 0.0138
b Lymphomas & solid tumors, adjusted P value = 0.0071
c Lymphomas & solid tumors, adjusted P value = 0.0120
dLymphomas & solid tumors, adjusted P value < 0.05
e CNS tumours & solid tumors, adjusted P value = 0.0206
f Lymphomas & solid tumors, adjusted P value = 0.0048
g Lymphomas & CNS tumors, adjusted P value < 0.05
Considering inflammatory biomarkers according to the extent of cancer (localized or advanced disease), it was found that NLR, SIRI, CRP, and procalcitonin levels were higher in patients with advanced disease (P values: 0.0349, 0.0175, 0.0006, and 0.0024, respectively) (Table 3 and Figure 2). There was no significant difference between the counts of leukocyte, neutrophil, lymphocyte, monocyte, eosinophil, basophil, and platelet, hemoglobin levels, erythrocyte sedimentation rate, procalcitonin levels, PLR, LMR, SII index, and HALP score between the patients with localized or advanced disease (all P values > 0.05).
| Parameters | Localize, (n = 46) | Advanced, (n = 51) | P Value |
|---|---|---|---|
| Mdn leukocyte counts, (/µL) | 7675 | 8700 | 0.23 |
| (Q1-Q3) | (5800-10950) | (6800-11200) | |
| Mdn neutrophil counts, (/µL) | 4050 | 4900 | 0.06 |
| (Q1-Q3) | (3275-5325) | (3500-7500) | |
| Mdn lymphocyte counts, (/µL) | 2500 | 2100 | 0.16 |
| (Q1-Q3) | (1900-3225) | (1510-3500) | |
| Mdn monocyte counts, (/µL) | 600 | 700 | 0.08 |
| (Q1-Q3) | (400-778) | (550-820) | |
| Mdn eosinophil counts, (/µL) | 100 | 100 | 0.66 |
| (Q1-Q3) | (73-200) | (0-220) | |
| Mdn basophil counts, (/µL) | 0 | 0 | 0.82 |
| (Q1-Q3) | (0-100) | (0-100) | |
| Mdn platelets count, (/µL) | 348500 | 410000 | 0.07 |
| (Q1-Q3) | (290250-436500) | (311000-510000) | |
| Mdn hemoglobin levels, (g/dL) | 12.6 | 11.3 | 0.06 |
| (Q1-Q3) | (11.1-13.3) | (10.2-13.1) | |
| Mdn neutrophil-to-lymphocyte ratio | 1.86 | 2.48 | 0.0349 |
| (Q1-Q3) | (0.93-3.0) | (1.13-5.27) | |
| Mdn lymphocyte-to-monocyte ratio | 4.09 | 3.5 | 0.0543 |
| (Q1-Q3) | (2.94-6.1) | (1.58-5.16) | |
| Mdn platelet-to-lymphocyte ratio | 146.4 | 170 | 0.16 |
| (Q1-Q3) | (117.4-201.3) | (112.1-314.4) | |
| Mdn systemic immune-inflammatory index | 32.13 | 27.6 | 0.99 |
| (Q1-Q3) | (17.81-51.34) | (19.11-49.44) | |
| Mdn systemic inflammatory response index | 1040 | 1533 | 0.0175 |
| (Q1-Q3) | (446.0-1531.0) | (736.8-3933.0) | |
| Mdn HALP score | 36.59 | 32.9 | 0.56 |
| (Q1-Q3) | (19.72-54.79) | (19.37-50.05) | |
| Mdn C-reactive protein, (mg/dL) | 0.35 | 1.19 | 0.0006 |
| (Q1-Q3) | (0.16-1.0) | (0.29-8.83) | |
| Mdn erythrocyte sedimentation rate | 19 | 24 | 0.15 |
| (Q1-Q3) | (7-33) | (9-54) | |
| Mdn, procalcitonin, (µg/L) | 0.05 | 0.15 | 0.0024 |
| (Q1-Q3) | (0.05-0.11) | (0.06-0.34) |
Abbreviations: Mdn, median; Q1, quartile 1; Q3, quartile 3.
Regarding the inflammatory biomarkers of the alive and dead patients, only the patients who died had significantly higher HALP score (P = 0.0472) (Table 4 and Figure 2). There was no statistical difference between the counts of leukocyte, neutrophil, lymphocyte, monocyte, eosinophil, and platelet, hemoglobin levels, erythrocyte sedimentation rate, CRP levels, NLR, LMR, PLR, SII index, and SIRI between alive and dead patients (all P values > 0.05).
| Parameters | Alive Patients, (n = 81) | Dead Patients, (n = 16) | P Value |
|---|---|---|---|
| Mdn leukocyte counts, (/µL) | 8450 | 8400 | 0.78 |
| (Q1-Q3) | (6550-10750) | (5173-11725) | |
| Mdn neutrophil counts, (/µL) | 4700 | 3960 | 0.84 |
| (Q1-Q3) | (3470-6595) | (2800-8793) | |
| Mdn lymphocyte counts, (/µL) | 2200 | 2050 | 0.48 |
| (Q1-Q3) | (1800-3200) | (1353-3650) | |
| Mdn monocyte counts, (/µL) | 600 | 770 | 0.17 |
| (Q1-Q3) | (500-800) | (525-1200) | |
| Mdn eosinophil counts, (/µL) | 100 | 150 | 0.92 |
| (Q1-Q3) | (45-200) | (0-280) | |
| Mdn basophil counts, (/µL) | 10 | 0 | 0.0218 |
| (Q1-Q3) | (0-100) | (0-23) | |
| Mdn platelets count, (/µL) | 377000 | 363500 | 0.72 |
| (Q1-Q3) | (316000-465500) | (219250-618000) | |
| Mdn hemoglobin levels, (g/dL) | 12.3 | 11.2 | 0.39 |
| (Q1-Q3) | (10.6-31.2) | (9.8-13.4) | |
| Mdn neutrophil-to-lymphocyte ratio | 2 | 1.95 | 0.74 |
| (Q1-Q3) | (1.1-3.57) | (0.74-5.19) | |
| Mdn lymphocyte-to-monocyte ratio | 4 | 2.6 | 0.13 |
| (Q1-Q3) | (2.78-6.0) | (1.33-5.51) | |
| Mdn platelet-to-lymphocyte ratio | 149.2 | 147.4 | 0.94 |
| (Q1-Q3) | (111.7-221.5) | (117.6-247.4) | |
| Mdn systemic immune-inflammatory index | 29.75 | 29.02 | 0.88 |
| (Q1-Q3) | (18.97-49.41) | (13.86-59.89) | |
| Mdn systemic inflammatory response index | 1225 | 880.8 | 0.98 |
| (Q1-Q3) | (601.4-2283.0) | (454.9-5533) | |
| Mdn HALP score | 31.31 | 52.12 | 0.0472 |
| (Q1-Q3) | (17.55-47.33) | (32.34-63.09) | |
| Mdn C-reactive protein, (mg/dL) | 0.6 | 2.43 | 0.23 |
| (Q1-Q3) | (0.22-1.66) | (0.17-9.85) | |
| Mdn erythrocyte sedimentation rate | 21 | 15 | 0.98 |
| (Q1-Q3) | (10-42) | (3-61) | |
| Mdn, procalcitonin, (µg/L) | 0.06 | 0.21 | 0.0094 |
| (Q1-Q3) | (0.05-0.23) | (0.09-0.48) |
Abbreviations: Mdn, median; Q1, quartile 1; Q3, quartile 3.
5. Discussion
Although cancer is one of the major health problems worldwide, unlike adults, it is rare in childhood. In children, while cancer is the second most common cause of death in developed countries, it ranks fourth in developing countries. The improvement in overall survival rates in the last three to four decades is satisfactory. In this population, the main factors affecting overall survival rates are pathological diagnosis, stage (in some cancers, localized, locoregional, or metastatic), and age at diagnosis (2). There are also prognostic factors specific to the type of cancers in children. Especially in adult cancers, the inflammatory biomarkers showing biological inflammatory status have been commonly used lately. Some of these inflammatory biomarkers are SIRI, SII index, NLR, PLR, CRP-albumin ratio, and HALP score (5-9). There is little experience with the use of these inflammatory biological markers in childhood (10-12). Herein, the aim of our study was to evaluate the importance of the biological inflammatory markers, including SII index, SIRI, and HALP score in children with cancer.
There are studies showing that increased leukocyte count is associated with poor prognosis in cancer patients, especially leukemia (17, 18). The studies on the importance of leukocyte count in malignant diseases other than leukemia in children are few. Logically, given that leukocyte counts increase in inflammation, higher leukocyte counts in cancer patients than in healthy controls can be expected. The prognostic significance of the leukocyte count has long been known (19, 20). In our patients, including all childhood cancers except leukemia and retinoblastoma, although the leukocyte count was higher than the control group, the difference was not statistically significant. However, when the leukocyte subgroups were examined, the neutrophil and monocyte counts of the patients were higher, and the lymphocyte counts were statistically lower than the control group. Especially the increase in the neutrophil count can be explained by inflammation and cytokines in cancer. Lymphopenia is expected to be seen in Hodgkin lymphoma or Langerhans cell histiocytosis. Lymphocyte count has prognostic significance, especially in Hodgkin lymphoma.
Recently, numerous studies have focused on the ratio of leukocytes subgroups. The inflammatory biological markers with prognostic values include NLR, PLR, and LMR (12, 20-27). In our study, we found that patients' neutrophil, monocyte, and platelet counts and NLR, PLR, and SIRI values were statistically higher than healthy volunteers, while lymphocyte and basophil counts, hemoglobin level, and LMR were low. The elevation in neutrophil and platelet counts is a reflection of the inflammatory process in cancer. The decrease in lymphocyte count is possibly associated with immunological events. Statistical differences in NLR, PLR, LMR, and SIRI are associated with changes in lymphocyte, neutrophil, and platelet counts. In cancer patients, low hemoglobin level is well known, and the reason is multifactorial. All these changes can be attributed to the body's response to cancer, similar to inflammation and wound healing (28).
Studies on biological inflammatory markers in children have mostly been done on tumors of the CNS (20, 24-26). Preoperative leukocyte count, mean platelet volume, and NLR ratio values of children with tumors of the CNS were found to be higher than healthy volunteers. However, no association between these parameters and survival rates has been reported (20). Another study compared children with medulloblastoma and pilocytic astrocytoma. While they found the lymphocyte count to be low in patients with medulloblastoma, NLR was high as a reflection of low lymphocyte count (24). In another study, there was a correlation between tumor grade and neutrophil count and NLR. Li et al. (26) assessed patients with medulloblastoma and found that while NLR and PLR had prognostic significance in univariate analysis, MLR had no prognostic significance. In multivariate analysis, NLR and PLR were found to have prognostic significance, as well.
These biological inflammatory markers were also studied in lymphomas (12, 27, 29). The NLR, MLR, PLR, and red blood cell distribution width of children with lymphoma were higher than children with reactive lymphadenopathy. The authors suggested that these markers may be useful to determine which patients with lymphadenopathy should be referred to the advanced center at an early stage for biopsy (12). In children with Hodgkin lymphoma, the presence of lymphopenia and leukocytosis adversely affect survival (29). High NLR is a prognostic factor in both univariate and multivariate analyses in Hodgkin lymphoma (27).
In children with solid tumors, high NLR affects both overall survival and event-free survival in univariate analysis, whereas only overall survival is affected in multivariate analysis (22). In another study on children with sarcoma, low lymphocyte count, improvement in late lymphocyte count, high NLR and PLR in osteosarcoma, lymphocyte count, late recovery in lymphocyte count, high NLR in rhabdomyosarcoma, and lymphocyte count in Ewing sarcoma were found to have a prognostic significance (23).
In our study, when we classified the diagnoses as lymphoma, tumors of the CNS, and solid tumors, it was found that there were differences between lymphocyte count, NLR, LMR, PLR, SII index, SIRI, and CRP values. These differences were found between lymphoma and solid tumors, except for the SII index. Regarding the SII index, the difference was observed between the tumors of the CNS and solid tumors. This observation may explain the main reason for this change, especially the low lymphocyte count in lymphomas. Additionally, it was found that patients with advanced disease had higher NLR, SIRI, CRP, and procalcitonin levels than patients with localized disease. This can be explained by the possibility of more intense inflammation in those with advanced disease. Finally, in our study, while the HALP score and procalcitonin values of the patients who died were higher than the patients who were alive, interestingly, the basophil counts were found to be lower. We could not explain the low number of basophils, and this brings to mind the question that whether it is a coincidence or not.
Considering our obtained findings, the lymphocyte count can be the main determining factor in these inflammatory biomarkers, as Vasquez et al. (23) emphasized, which can be explained by the relationship between cancer and inflammation.
The most important limitation in our study was that we could not perform survival analyses due to the short follow-up periods. However, we think that the comparison of alive and dead patients can give an idea, albeit a little.
In conclusion, these inflammatory biomarkers, which have no extra cost and can be easily calculated from the complete blood count, can be used in clinical settings. Another point is that careful monitoring of lymphocyte counts, presence of lymphopenia according to age, and lymphopenia recovery time at the diagnosis may also be beneficial for determining the outcome of treatment.