1. Background
Cleft lip and cleft palate are the fourth most common congenital defect and the most common genetic defect of the face. Cleft lip and cleft palate are the primary oral-facial deformity with a prevalence of about 1 - 2.2 per thousand births. The treatment of this anomaly is performed at 9 - 18 months of age using different surgical techniques to separate oral and nasal cavities (1). These two anomalies account for 65% of head and neck abnormalities seen in isolation or as part of a syndrome (syndromic).
Bleeding that requires operative homeostasis is the most common postoperative complication after palatoplasty surgery in patients with cleft palate. This complication usually appears when the patient is undergoing surgery and before extubation or when the patient is in the recovery room. Common methods for stopping these postoperative hemorrhages are patient transfer to the operating room, reintubation, and preoperative homeostasis (2).
The mouth and face are vascular areas that can bleed profusely during surgery and subsequently create the need for blood transfusions. Although these hemorrhages are often manageable, they can lead to the presence of blood and degraded field quality during surgery. The various amount of bleeding during surgery can cause impaired surgeon vision and prolonged surgery and sometimes requires blood transfusions. Various methods are used to reduce bleeding in the surgical field, including head posture, controlled breathing, and medication to control hypotension, such as beta-blockers, nitro glycerol, and calcium channel blockers (3).
Another method of reducing bleeding in surgery is using antifibrinolytic agents such as tranexamic acid (TXA), prothrombin, and aminocaproic acid. The administration of these drugs around the operation stabilizes small and numerous clots at the surgical site.
Tranexamic acid is a synthetic lysine derivative that inhibits fibrinolysis by binding to and inhibiting plasminogen. It competitively inhibits plasminogen activation and prevents its conversion to plasmin and fibrin breakdown, resulting in blood clot stability and reduced bleeding (4, 5). This drug has been used to control bleeding in various surgical fields, including orthopedics, obstetrics, and gynecology, and has had a good effect in reducing bleeding and blood transfusions (6, 7). Based on previous studies in a clinical trial and systematic review, TXA has not been shown to increase surgical side effects such as thromboembolic events (8-10).
The half-life of this drug is 2 hours from the administration of 1 gram of intravenous drug.
Urinary excretion is the primary means of tranexamic acid elimination, with > 95% of an administered dose excreted in the urine as unchanged parent drug contraindications: Known allergy to TXA, intracranial bleeding, known defective color vision, history of venous or arterial thromboembolism, or active thromboembolic disease (3).
This drug is well tolerated, and its gastrointestinal side effects include nausea, vomiting, and anorexia, most commonly seen orally. If the drug is injected quickly, it may cause hypotension. In rare cases, thrombotic events have been observed. Drug sensitivity to facial skin rash and blistering lesions such as itching have also been reported, mostly with long-term use of the drug. This complication also disappears within a few days after stopping the drug.
Haddadi et al. evaluated the effect of a prophylactic dose of TXA on bleeding rates in mandibular surgeries. They reported that intravenous TXA after anesthesia induction reduced the bleeding rate during traumatic jaw surgeries (3).
Alimian and Mohseni conducted a study on the effect of intravenous TXA on the amount of hemorrhage and the quality of the surgical field during sinus endoscopy. They concluded that TXA effectively reduced the bleeding and improved surgical field surgery (11).
2. Objectives
This study aimed to investigate the effect of preoperative intravenous TXA on hemorrhage in patients undergoing cleft palate reconstruction.
3. Methods
This clinical trial was performed on patients who were candidates for cleft palate surgery in the Khatam Al-Anbia Hospital, Zahedan, Iran, in 2018. Patients with the indication of palatoplasty with congenital cleft lip (with or without lip cleft) were included in the study. The study population was 60 patients divided into a TXA receiving group and a control group, each with 30 patients.
The exclusion criteria were as follows: Preoperative hemoglobin less than 10 mg/dL or platelets less than 100,000/mm3, coagulopathy provoked by a known cause or a systemic disease or a history of excessive bleeding, history of bleeding disorders in a first-degree family, use of drugs interfering with the coagulation process (platelet inhibitors or anticoagulants), palatoplasty as a candidate for the second operation to correct the oronasal fistula, known sensitivity to tranexamic acid, and history of hematuria.
The patients were randomly assigned into the case or control group using a randomized block. The patients' demographic information (age, gender, and weight) and study variables (bleeding rate, Hb, PT, PTT before and after surgery, and duration of surgery) were recorded using pre-designed data collection forms. Data were collected, and the intervention was performed during surgery. After considering the inclusion and exclusion criteria, eligible patients received the intervention in the operating room. In the operating room, each patient underwent general anesthesia and intubation within the nasotracheal tube after establishing an intravenous route.
A double-blind face was injected after induction of anesthesia and before incision at a dose of 10 mg/kg of TXA within 15 minutes and 1 mg/kg/h by infusion until surgery in the case group or normal saline in the control group. The amount of bleeding through the eye (12-14) was based on the number of blood-stained gases, and the amount of bleeding in the suction was as follows: The number of 4 × 4-inch blood-stained gases and the volume of suction blood recorded separately for each patient. Each 4 × 4-inch blood-soaked gas contained 10 mL of blood. The number of blood-stained gases was multiplied by 10 mL, and the result was added to the volume of the suctioned blood. As the obtained volume included the volume of washing liquids, it also provided the range of surgery and reduced the volume of fluid consumed; the result was equal to the amount of actual blood loss of the patient (15, 16).
The patients were monitored regarding bleeding in the ward after surgery until discharge, and their information was collected. Also, the hemoglobin level was measured 6 hours after surgery. The data were described using descriptive statistics, including frequency, percentage, and mean. Also, an independent t-test was used to compare the quantitative data between the groups, and the chi-square test and Fisher's square or exact test were used to compare the qualitative data between them.
The ethics committee of Zahedan University of Medical Sciences approved our study with the ethics code IR.ZAUMS.REC.1394.300. A written consent to participate in the study was obtained from each patient.
4. Results
In this study, 60 patients (30 patients in the TXA receiving group and 30 patients in the control group) undergoing cleft palate surgery were evaluated. The mean age of the patients was 21.42 ± 9.46 months, and of the 60 patients, 31 were boys and 29 were girls. Also, the mean age of patients was 19.70 ± 7.87 months in the TXA receiving group and 23.13 ± 10.67 months in the control group (Table 1). Regarding gender, patients were 15 girls and 15 boys in the control group and 16 boys and 14 girls in the TXA receiving group. There was no statistically significant difference in the mean age between the case and control groups (P-value = 0.162).
| Group | Age | P-Value |
|---|---|---|
| Tranexamic acid receiving | 19.70 ± 7.87 | 0.162 |
| Control | 23.13 ± 10.67 |
Table 2 compares the mean bleeding in the control and TXA receiving groups. Mean bleeding was 11.73 ± 4.42 milliliters in the TXA receiving group and 17.36 ± 4.99 milliliters in the control group, which was significantly different (P-value = 0.001).
| Group | Mean ± SD | P-Value |
|---|---|---|
| Tranexamic acid receiving | 11.73 ± 4.42 | 0.001 |
| Control | 17.36 ± 4.99 |
Table 3 compares the mean duration of surgery and anesthesia between the control and TXA receiving groups. As can be seen in the table, this difference was statistically significant (P-value = 0.002).
| Group | Average Duration (Min) | P-Value |
|---|---|---|
| Tranexamic acid receiving | 41.90 ± 8 | 0.002 |
| Control | 49.93 ± 11.37 |
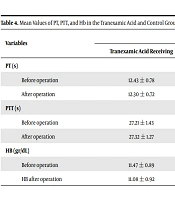
The mean values of PT, PTT, and Hb in the TXA receiving and control groups did not differ much before and after surgery, which is also shown in Table 3.
Table 4 shows the mean values of PT, PTT, and Hb in the TXA receiving and control groups before and after surgery.
| Variables | Group | P-Value | |
|---|---|---|---|
| Tranexamic Acid Receiving | Control | ||
| PT (s) | |||
| Before operation | 12.43 ± 0.78 | 12.31 ± 0.89 | 0.572 |
| After operation | 12.30 ± 0.72 | 12.29 ± 0.78 | 0.932 |
| PTT (s) | |||
| Before operation | 27.21 ± 1.43 | 27.12 ±1.27 | 0.798 |
| After operation | 27.32 ± 1.27 | 27.45 ± 1.11 | 0.692 |
| HB (gr/dL) | |||
| Before operation | 11.47 ± 0.89 | 11.52 ± 0.98 | 0.849 |
| HB after operation | 11.08 ± 0.92 | 10.82 ± 0.83 | 0.121 |
5. Discussion
This study aimed to investigate the effect of preoperative intravenous TXA on hemorrhage in patients undergoing cleft palate reconstruction surgery in the Khatam Al-Anbia Hospital. The results showed that TXA significantly reduced the amount of bleeding and the duration of surgery in cleft palate surgery.
About 70% of cases are cleft lip, and palate is multifactorial. Clefting of the lip with or without palate is the most common congenital craniofacial anomaly with the global prevalence estimated at 1 in 700 live births.
Various abnormalities have been reported, from 0.77 to 3.37 cases per thousand births. According to studies done in Iran, the incidence rate of cleft lip and palate is one per thousand live births, which is less than that in other countries in the Middle East, Asian countries, and American countries. Cleft palate and cleft lip are associated with many other problems (17), including autologous diseases, speech and language problems (speech delay, pronunciation disorders, and velopharyngeal disability), dental deformities (poor pairing, lack of or excess teeth, and deformity), facial development defects, and psychosocial problems in children and their families. The primary treatment for cleft lip and cleft palate disorders is surgery. One of the main complications of this surgery is bleeding. Tranexamic acid is a compound shown in some studies to reduce bleeding in cleft lip and palate surgery (18). According to the results of this study, the mean bleeding rate was significantly lower in the TXA receiving group than in the control group. In Albirmawy et al.'s study, the mean amount of blood lost in adenoidectomy was significantly lower in the TXA receiving group than in the control group. In this study, the duration of surgery in the TXA receiving group was significantly reduced compared to the placebo group (19).
Arantes et al. evaluated the efficacy of TXA in reducing intraoperative bleeding in palatoplasty. They reported that the amount of blood lost in the TXA receiving group decreased by 11.9% compared to the control group, although not significantly different. However, this finding is not consistent with the results of our study (4).
In this study, the mean duration of surgery and anesthesia was significantly less in the TXA receiving group than in the control group.
Ghomeishi et al. conducted a study on 66 candidates for endoscopic sinus surgery in Ahvaz. They observed that the mean duration of surgery was less in the TXA receiving group than in the control group, although not significantly different (20).
However, our results showed that the mean values of PT, PTT, and Hb were not significantly different between the TXA receiving and control groups before and after surgery. In Durga et al.'s study in India on 65 children who underwent cleft palate surgery, the mean amount of hemoglobin was not significantly different between the TXA receiving and control groups before and after surgery (21).
5.1. Conclusions
Our study showed that TXA significantly reduced the amount of bleeding and the duration of surgery in cleft palate surgery but had no significant effect on PT, PTT, and hemoglobin levels before and after surgery.