1. Background
Tuberculosis, as an infectious disease, is a worldwide problem (1). Bacille Calmette –Guerin (BCG) vaccine is the only available vaccine used worldwide. It is an attenuated Mycobacterium bovis strain (2, 3). World Health Organization (WHO) recommends BCG vaccination for all infants in countries with endemic tuberculosis (3-6). According to different studies, the efficacy of BCG vaccine against disseminated tuberculosis and meningitis is about 80% and against pulmonary tuberculosis (TB) is about 50% (6, 7). BCG vaccine is injected into about 100 million infants each year (7, 8). According to Iran’s vaccination protocol, BCG vaccine is injected into all newborns at the time of birth (2, 9). Although the efficacy of BCG vaccination against tuberculosis is noteworthy, it also has some risks (10). It is a safe vaccine for those with an immunocompetent system, but it can produce some local or systemic complications in immunosuppressed children, such as SCID and CGD (4, 11, 12). Some early vaccine reactions, including erythema, induration, papule, ulcer, and abscess, may present at the injection site. Following intradermal injection of the vaccine, a papule with a maximum diameter of 10 to 20 mm is usually observed after six weeks. A small ulcer with some purulent discharge may occur at the injection site, which heals within the 10th week; a small scar may follow this in most patients. Some possible risk factors involved in producing side effects include the age of the patient, vaccine dose, BCG strain, inoculation technique, and the site of injection (13).
Some more serious delayed vaccine reactions include osteomyelitis, lymphadenitis, prolonged local ulceration for more than six months, and also disseminated BCG (8, 11). The rate of local problems associated with BCG vaccination is about 1 in 2,500 injections, whereas it is about 1 in 100,000 for disseminated BCG disease (8). Lymphadenitis is the most common side effect of BCG vaccination (6, 9). According to a cohort study in India, disseminated BCG infection following vaccination is a strong predictor of a suppressed immune system. According to the authors, even sustained localized adenitis may be a sign of a defect in the immune system (4). The majority of previous studies have focused on the different side effects of BCG vaccine in immunocompromised children, and there are a few studies on the defects of the immune system in apparently healthy children presenting with BCG adenitis (2-5, 10).
2. Objectives
This study aimed to evaluate the immunoglobulin status, lymphocyte count, and PPD size in children with BCG adenitis.
3. Methods
The cases included 40 children with BCG lymphadenitis referred to Taleghani hospital in Gorgan city in 1396 who were compared to 40 healthy children as the control group. In this study, there were 40 patients with lymphadenitis of whom 24 were boys (60%) and 16 were girls (40%), and 22 boys (55%) and 18 girls were in the control group (45%). The children were age- and sex-matched in both groups. All the participants had received the intradermal injection of 0.05 ml of BCG vaccine on the left arm at birth. Exclusion criteria were hepatosplenomegaly, lymph nodal involvement in other regions, severe anemia, chronic disease, and clinical evidence of disseminated BCG infection.
Parental consent was taken after explanation of the study and the patient history was obtained. The venous blood samples, including 5 mL citrated whole blood, were used for the measurements of lymphocyte counts and antibody levels. The serum samples were separated and stored at -70°C. After thawing the serum samples with the standard method, IgG, IgM, IgA, and IgE antibody levels were measured by the ELISA method. Avecina ELISA equipment was used to assess the IgE levels. The assessment of other immunoglobulins was done with the MINIEPH nephelometry equipment from Biding soft company. Complete blood counts (CBC) were evaluated with the Simex coulter immediately.
Purified protein derivative (PPD) test was done simultaneously with the injection of 0.01 ml or 5 IU of PPD-5 using the Mantoux method on the volar aspect of forearm by trained personnel. The result was observed by an expert after 48 hours by the measurement of induration via the ball-points pen method. The collected data were analyzed using the SPSS software version 18. Median, mean, standard deviation (SD), and percentage were used for data description. The P-value of less than 0.05 was considered significant. Independent t-test or the non-parametric Mann-Whitney U test was used to compare quantitative variables and immunoglobulin levels in the two groups, and chi-square test to compare the percentage of immunoglobulin deficiency in each group.
4. Results
Eighty children participated in the study, including 40 with tuberculous lymphadenitis and 40 healthy children who presented for routine testing. In the lymphadenitis group of 40 children, the number of boys was 24 (60%) and of girls was 18 (45%). There were 22 boys (55%) and 18 girls (45%) in the control group, and there was no statistically significant difference between the groups in terms of gender (P-value = 0.65) (Table 1). The mean age in the case group was 19.2 months. (SD = 10.677) and that of the control group was 21.85 months (SD = 22.456). Children from 2 months to 6 years of age were entered into the study. In this study, there was no statistically significant difference between the two groups in terms of age (P-value = 0.61, Table 1).
| Variables | Case | Control | P-Value |
|---|---|---|---|
| Sex | 0.65 | ||
| Boy | 24 (60) | 22 (55) | |
| Girl | 16 (40) | 18 (45) | |
| Age (mo) | |||
| < 24 | 30 (75) | 28 (70) | 0.61 |
| ≥24 | 10 (25) | 12 (30) | 0.004 |
| PPD (mm) | 5.86 ± 5.44 | 3.04 ± 2.32 | |
| Growth | |||
| Normal | 33 (82.5) | 36 (90) | 0.518 |
| Mild FTT | 7 (17.5) | 4 (10) | 0.51 |
| Lymphocyte | 5480 ± 2035 | 5118 ± 2793 | |
| Lymphopenia | |||
| Positive | 5 (12.5) | 0 (0) | 0.06 |
| Negative | 35 (87.5) | 40 (100) | 0.52 |
| WBC | 9810 ± 2725 | 9383 ± 3425 | |
| IgA | |||
| Mean ± SD | 0.69 ± 0.402 | 0.96 ± 0.262 | 0.001 |
| Normal | 35 (87.5) | 32 (80) | 0.366 |
| High | 5 (12.5) | 8 (20) | |
| IgG | |||
| Mean ± SD | 8.35 ± 3.64 | 8.43 ± 2.96 | 0.92 |
| Normal | 33 (82.5) | 39 (97.5) | 0.025 |
| High | 7 (17.5) | 1 (2.5) | |
| IgM | 0.016 | ||
| Mean ± SD | 0.97 ± 0.359 | 1.14 ± 0.265 | |
| Normal | 40 (100) | 40 (100) | |
| IgE | |||
| Mean ± SD | 86.52 ± 151.8 | 86.66 ± 146.39 | 0.762 |
| Normal | 28 (70) | 29 (72.5) | 0.806 |
| High | 12 (30) | 11 (27.5) |
Variables in the Case and Control Groups a
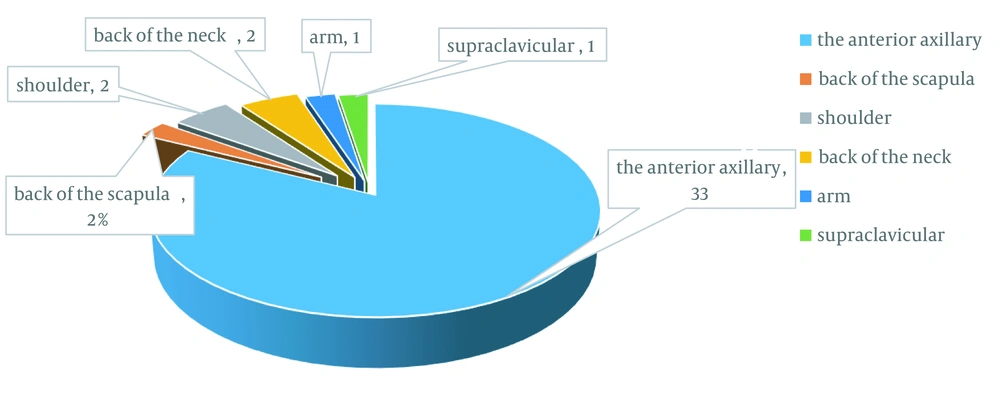
The two groups were compared for height, weight, and head circumference. According to Gomez’s classification for growth, development, and malnutrition, 33 children (82.5%) in the case group and 36 (90%) in the control group had normal growth patterns, and only seven children (17.5%) in the case group and four in the control group (10%) had mild growth retardation, which was not statistically significant (P-value = 0.518, Table 1). Lymphadenitis was ipsilateral to the injection site in all 40 studied children. The distribution of lymphadenitis was as follows:
Thirty-three cases on the anterior axillary region (82%), 1 (2.5%) in posterior scapula, 2 (5%) on the anterior shoulder, 2 (5%) on the posterior cervical region, 1 (2.5%) on anterior arm, and 1 (2.5%) on the supraclavicular region (Figure 1). Abscess of the lymphadenitis site was observed in 10 cases (25%) out of 40 studied children. The mean number of white blood cells (WBC) and lymphocytes in the case group was 9810 ± 2725 and 5480 ± 2035, respectively, whereas it was 9,383 ± 3,425 and 5,118 ± 2,793 in the control group, respectively (P-value = 0.52, P-value = 0.51, Table 1).
According to independent T-test, the mean lymphocyte and WBC in the two groups were not statistically different. In this study, five patients (12.5%) under the age of one year had lymphopenia (lymphocyte count < 3,000), but no lymphopenia was observed in the control group. On the other hand, none of the participants had lymphocyte counts below 1,000. Leukopenia was observed in none of the participants (Table 1). We obtained the following results when comparing the immunoglobulin (Ig) levels (IgM, IgG, IgA, and IgE) between the two groups:
The mean IgA level in the lymphadenitis group was significantly lower than the control group (0.69 mg/dL, SD = 0.402, and 0.96 mg/dL, SD = 0.262, respectively) by the independent t-test (P = 0.001). We also made a classification for IgA level based on a range defined by the laboratory and divided the IgA level into three categories high, normal, and low levels, and compared the two groups of the study with respect to these levels. No one had a low IgA level (0%), 35 (87.5%) had normal IgA level, and 5 (12.5%) had high levels in the group with lymphadenitis. The serum IgA level was low in no one (0%), normal in 32 (80%), and high in 8 (20%) in the control group.
There was no significant difference between the two groups of study in the low, normal, and high IgA levels, according to Mann-Whitney U test (P = 0.366). The mean IgM level in the lymphadenitis group was significantly lower than the control group (0.97 mg/dL, SD = 0.359, and 1.14 mg/dL, SD = 0.265, respectively) by the independent t-test (P = 0.016). Both groups had normal IgM levels (Table 1). There was no difference in the mean IgG levels between the two groups, but IgG levels was higher in the control group when using the previous classification of normal and high range using the non-parametric Mann-Whitney U test (P = 0.025, Table 1).
The mean IgE level in the two groups was not different according to Independent t-test (P = 0.762), and there was also no statistical difference when using non-parametric Mann-Whitney U test (P = 0.806) in the low and normal levels of this antibody (Table 1). The location of BCG scar was at the correct site, except for one case in the lymphadenitis group. The size of induration following BCG vaccination was greater in the lymphadenitis group than the control group, according to Mann-Whitney U test (P = 0.001, Table 2). The mean age of children with respect to induration size was as follows: < 5 mm, 19.5 months; 5 - 10 mm, 15.3 months; 10 - 15 mm, 30 months; > 15 mm, 57.3 months.
| Variables | PPD (mm); No. (%) | P-Value | |||
|---|---|---|---|---|---|
| < 5 | 5 - 10 | 10 - 15 | > 15 | ||
| Case | 21 (52.5) | 14 (35) | 1 (2.5) | 4 (10) | 0.001 |
| Control | 35 (87) | 4 (10) | 1 (3) | 0 (0) | |
| Total | 56 (70) | 18 (22.5) | 2 (2.5) | 4 (5) | |
The size of PPD Indurations in the Case and Control Groups
5. Discussion
According to different studies, BCG vaccination has decreased the incidence of miliary tuberculosis and tuberculous meningitis, whereas some different studies have reported serious reactions following BCG vaccination, especially in children with primary immunodeficiency (6, 7). Evaluation of the immune system, especially in children suffering from vaccine complications, can help identify the defects of the immune system at an early stage. This is specifically helpful in our country where the BCG vaccination is routinely performed according to the countries vaccination protocol, and no screening for immunodeficiencies is done before that, so we performed this study to evaluate the immune status of children who developed only lymphadenitis following the BCG vaccination.
According to different studies in Iran, the prevalence of lymphadenitis is between 0.54 - 5.8 percent (9, 11, 14, 15). These differences may be due to differences in vaccine strain, vaccine maintenance, and the injection technique (13). The mean age in the case group was 19.2 months. In a study by Zamora et al., the mean age for lymphadenitis occurrence was 4 months (1 - 9 months) (16). In another study by Behjati et al., the incidence of lymphadenitis was 53.8% within the first 1 to 4 months of age and 42.3% within 4 to 6 months of age (9). According to Barari-Savadkouhi et al., most cases of lymphadenitis develop in the first 4 months of life (11). In the study by Satyanarayana, the mean age for the occurrence of lymphadenitis was 9.3 months (17).
In this study, in accordance with other studies, the involvement of the ipsilateral axillary nodes was the most common lymph nodal involvement (9, 16, 17). There was no statically significant difference between the two groups in terms of growth parameters, including height, weight, and head circumference in this study. When evaluating the number of involved lymph node sites with the presence of FTT, 43% of children with FTT showed more than one site of lymph nodal involvement in comparison to 12% of children without FTT (P = 0.08). The authors of this article conclude that FTT and malnutrition can be considered important risk factors for the increase in the severity of lymphadenitis in children, which can be a matter for future studies.
There was no difference between the two groups in the mean lymphocyte and WBC counts when considering the immune status. Although five patients (12.5%) below one year had lymphopenia, there was no lymphopenia in the control group. Considering that the reported lymphopenia is only in children below one year, further studies are needed with a larger sample size. According to a study in Russia on 24 children with BCG adenitis or osteitis, the frequency of immunodeficiency was 92%. The study did not evaluate WBC and lymphocyte counts, which created bias because flow cytometry results should be interpreted by a prior CBC report (2).
In a study by Sevostyanova, serum IgA levels were low in 25% and not detectable in 33.3% of the subjects (2). Of the 8 patients with no detectable IgA, five patients showed low serum IgG levels, and three individuals had normal levels. Serum IgM levels were high in two children and normal in six children. IgG and IgM levels were normal in one child with no detectable IgA (2). In addition, Krasnoproshina LI showed 16.6% of cases had selective IgA deficiency, and 20.8% had hyper IgM syndrome (presenting with IgA and IgG deficiency) (2), which was not found in our study. In our study, the mean IgA level was significantly less in the group with lymphadenitis than the control group; however, in our study, the level of immunoglobulin A was in the normal and high range.
On the other hand, we only measured the Ig levels, unlike the investigators in Russia who did the measurement of immunoglobulin levels, flow cytometry, and identification of CD markers on lymphocytes. We found no significant difference in the mean IgG levels between the two groups in our study; however, a significant increase in IgG levels was found in the case group when we analyzed the data based on normal and high level range. The authors in Russia showed low serum IgG levels in 54% and high levels in 8% (2). The results of that study are somewhat contradictory, which may be plausible by the low number of cases.
In our study, we found significantly lower IgM levels in the lymphadenitis group compared with the control group; however, the serum IgM levels were within the normal range according to age. The study in Russia showed high IgM levels in 17% of patients and low levels in 4% (2). The children with lymphadenitis following BCG vaccination showed a larger size of induration following PPD test than the control group (5.44 mm versus 3.04 mm, respectively, P < 0.001). According to Alborzi et al. 2003, the mean size of induration between the lymphadenitis and the control group was 19.7 mm and 7.5 mm, respectively (18). According to 14 studies on Iranian children, the size of the induration was < 5 mm in 60%, 5 - 9 mm in 29.9%, and 10 mm or more in 8.5% (19).
5.1. Conclusions
Considering the normal immunoglobulin levels and CBC reports, although the probability of a primary immunodeficiency disorder was low in the cases of our study, further studies with a larger sample size and more specific investigations like flow cytometry and specific antibody response are needed.