1. Background
Sleep is a central component of brain development. A term child spends a significant period of the primary stages of his/her life developing structures, such as the hippocampus, pons, brainstem, and mesencephalon, while sleeping, even after birth; accordingly, the sensory nervous system is shaped (1).
Few studies have been conducted on neonates’ sleep-wake cycles (SWCs). However, they showed that neonates with a gestational age of 28 - 32 weeks follow an extremely exclusive sleep-wake rhythm in a way that they can sleep up to 22 hours per day, 70% of which is spent during active sleep. Disruption in these processes in preterm neonates (e.g., premature birth and long-term hospitalization in stressful neonatal wards) might negatively affect the formation of a coherent structure of the neurobehavioral system through physiological and biochemical interruptions since sleep can provide a period of time for the production of neurotransmitters which are active in this process. Sleep deprivation, both in the active phase [active/rapid eye movement (REM)] and the inactive phase (quiet/nonREM) for 2 to 4 hours per week, in a neonate in the 32nd week of pregnancy, along with lack of acceptable SWCs, will ultimately affect brain flexibility in adaptation and learning processes and can lead to health issues as a result of impaired functionality in other physiological activities (e.g., the occurrence of apnea, bradycardia, and impaired blood sugar homeostasis) (2, 3).
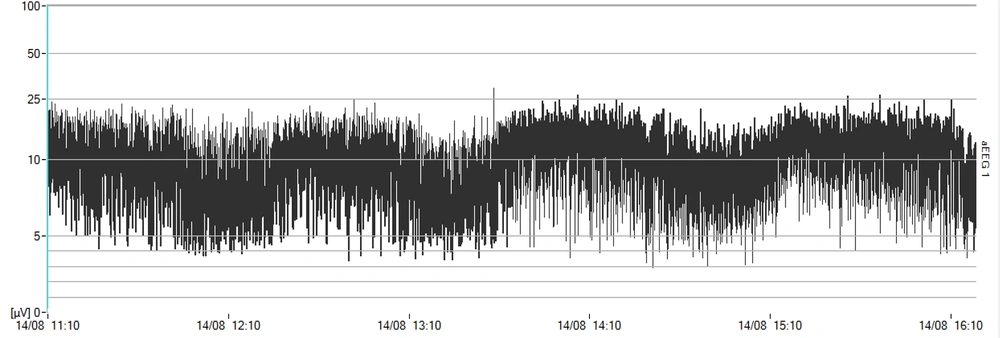
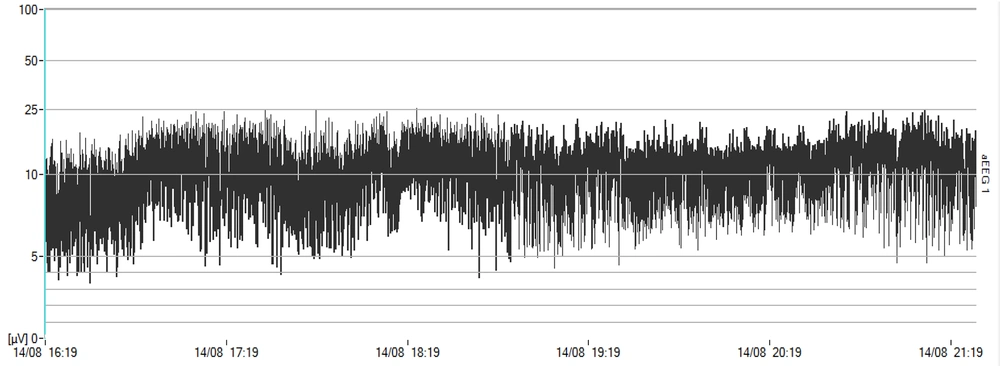
The development of amplitude-integrated electroencephalography (aEEG) technology has revolutionized the monitoring methods of a neonate’s sleep-wake cycle. While generating aEEG, the EEG signal is first amplified and then passed through a frequency-selective limiter to filter the EEG trace having signals with frequencies below 2 Hz and above 15 Hz. The EEG signal for aEEG is recorded from one channel (or two channels). Once these signals are compressed, they are displayed in the classic trace time unit, using which the aEEG, continuous activity, discontinuous activity, burst-suppression, and cyclical changes, such as SWCs, or even their quality, can be examined. During quiet sleep (QS), the band amplitude is maximized. When a neonate enters the active phase of his/her sleep cycle, it will reach its minimum due to the continuity in the electrical activity of trace amplitude. This pattern is distinguishable and identifiable in the trace (4).
Respiratory distress syndrome (RDS) is the most common pathology in neonates aged less than 32 weeks of gestation. The disease and its associated complications still account for a significant proportion of long-term mortality and morbidity among neonates. Although the survival of neonates with RDS has significantly improved in recent decades thanks to interventions, such as prenatal corticosteroid administration, surfactant replacement, and broader use of noninvasive mechanical ventilation, the reduction observed in the prevalence of morbidity has not been as significant as the reduction in the prevalence of mortality, making these neonates still prone to developing high-risk neurodevelopmental complications (5, 6).
For over four decades, nasal continuous positive airway pressure (nCPAP) has been known as the standard care in RDS management. The nCPAP improves the process of oxygenation in neonates with RDS by rehabilitating and maintaining lung volume, preventing atelectasis in lung structure, stabilizing the chest wall, improving pulmonary compliance, and reducing dynamic work of breathing (WOB). By increasing the efficacy of the ventilation/perfusion ratio, nCPAP can decrease intrapulmonary shunt. Furthermore, nCPAP can reduce airway resistance by creating a support framework in airways and preventing it from collapsing, which, along with reduced thoracoabdominal asynchrony, tachypnea, and intercostal retraction, can reduce WOB. In neonates suffering from decreased respiratory function due to poor muscle strength, nCPAP can reduce breathing difficulty by minimizing the pressure resulting from the heaviness of the chest wall structure. Observations have shown that, even in neonates experiencing recurrent central apnea, the gas stream that nCPAP sends to the upper respiratory tract can prevent such interruptions in the process of respiration only through its irritating effect on these areas. However, nCPAP shows a known mechanism in the prevention of obstructive sleep apnea by keeping the airways open (7).
The early uses of nasal intermittent positive pressure ventilation (NIPPV) were developed to control apnea of prematurity. Using NIPPV as the primary mode (when NIPPV is used in less than or equal to 2 hours after birth in a neonate with respiratory distress, which can also be accompanied by surfactant administration) has been around for some time in neonatal intensive care units. However, this approach indicates wide distribution in the treatment of RDS because, in the UK alone, the difference in usage of this approach as the primary mode shows a rise from 48% in the UK to 61% in Ireland (8).
Although it has always been emphasized that the mechanism of cerebral autoregulation (even in its undeveloped form) in combination with systemic mean blood pressure above 30 mmHg can be sufficient to support cerebral blood flow in preterm neonates, recent studies have shown that other factors, such as the level of pressure inside the chest, can affect not only the blood flow inside the skull but also the local oxygenation level of the brain. Therefore, it is known that interventions associated with respiratory support are the ones that are significantly related to specific changes in intrathoracic pressure (9, 10).
2. Objectives
Currently, technologies, such as near-infrared spectroscopy, functional magnetic resonance imaging, and aEEG, have helped monitor brain activities in developing neonates more than in the past and evaluate the impact of therapeutic interventions on these activities (11). With reference to the above-mentioned studies, the widespread use of NIPPV in neonatal wards, and the concerns which exist over the use of nonsynchronized respiratory support (12), it was decided to assess the quantity and quality of SWCs, which can represent physiologic brain activities, in two types of intervention, namely nCPAP and NIPPV respiratory support, in a randomized controlled trial (RCT) crossover study.
3. Methods
3.1. Design and Setting
The present RCT crossover study (in which all participants received all the interventions) was conducted on neonates with a gestational age of 28 - 32 weeks receiving nCPAP respiratory support at the 72nd hour of birth due to RDS in Shahid Beheshti hospital in Isfahan, Iran, within March 2018 to August 2020. The neonates entering this study were diagnosed with RDS with manifestations of tachypnea, intercostal retraction, nasal flaring, and grunting, along with chest X-ray associated with RDS, and had been administered surfactant during the first 2 hours of life. Survanta was administered in neonates with a fraction of inspired oxygen (FiO2) ≥ 30% and continuous distending pressure (CDP) ≥ 5 cm H2O in order to maintain peripheral oxygen saturation (SpO2) within the range of 89 - 95% in the right hand. Moreover, the neonates were provided with nCPAP support at the 72nd hour of birth to maintain SpO2 within the range of 89 - 95% with CDP within the range of 4 - 6 cm H2O and FiO2 within the range of 30 - 40% (13-15).
The exclusion criteria were perinatal asphyxia (characterized by a 5-minute Apgar score of 0 - 3, umbilical arterial pH less than 7, and bicarbonate of umbilical arterial less than 12 mEq/L), congenital anomalies, neonates not receiving surfactant or received it later than 2 hours after birth, neonates with evidence of patent ductus arteriosus in echocardiography, neonates receiving inotropic drugs, neonates with evidence of germinal matrix-intraventricular hemorrhage of grade III-IV in cranial ultrasound, and neonates suffering from air leakage processes (15-18).
This research study was registered on the Iranian Registry of Clinical Trials (reference No.: IRCT20120728010430N9).
3.2. Patients
Based on the primary purpose of this study, 27 neonates with the demographic parameters shown in Table 1 who met the criteria for entering the study at the 72nd hour of birth entered the research. The neonates in the study who were outside the active RDS period were monitored by cerebral function monitoring (CFM) for 24 hours (including two 12-hour periods).
| Variables | Values |
|---|---|
| Gestational age (week) | 29.4 ± 1.4 |
| Gender | |
| Male | 14 (51.8) |
| Female | 13 (48.1) |
| Weight (g) | 1255.5 ± 273.9 |
| Apgar score | |
| 1st minute | 5.6 ± 2.4 |
| 5th minute | 8.2 ± 1.8 |
| ROM ≥ 18 hours | 6 (22.2) |
| Maternal steroid administration | 27 (100) |
| Delivery | |
| C/S | 21 (77.7) |
| NVD | 6 (22.2) |
Abbreviations: SD, standard deviation; ROM, rupture of membranes; C/S, cesarean section; NVD, normal vaginal delivery.
a Values are expressed as mean ± SD or No. (%).
3.3. Intervention
First, the Olympic Medical CFM 6000 monitor (Natus Medical Incorporated, San Carlos, USA) was activated. Then, using NuPrep abrasive gel, the parietal areas on both sides of the cranium were rubbed, and the hydrogel electrodes were fixed in place (4). The neonates received nCPAP respiratory support using BabyFlow Injector (Draeger Medical, Lubeck, Germany) and Babylog 8000 plus ventilator (Draeger Medical, Lubeck, Germany). The neonates were monitored by aEEG for 12 hours while the study inclusion criteria were all met for administration for 12 hours with indicators consistent with entry into the study under aEEG. Then, specifications, including Ti = 0.5”, rate = 30 breaths/minute, and inspiratory positive airway pressure (IPAP) = expiratory positive airway pressure + 4 cm H2O, were defined for the ventilator. The mode was activated in IPPV mode. During the time NIPPV support was provided for the neonate, which was equivalent to 12 hours, monitoring was continued using aEEG, and CFM trends were recorded and stored to be reviewed later. During QS, the band amplitude of the CFM trace was maximal. The trace amplitude was minimal when the neonate entered the active phase of the sleep cycle due to continuity, high frequency, and reduced amplitude in electrical activity. This pattern was distinguishable in the trace (19, 20).
Oxygen saturation, respiration rate, and heart rate were monitored every hour. Blood gas and mean arterial pressure pulses were also monitored every 6 hours. The data obtained from 27 neonates were analyzed using SPSS software (version 18) through an independent t-test, Pearson correlation test, and Chi-square test with a confidence level of 95%, test power of 80%, and error of 0.37.
3.4. Main Outcome Measures
The primary objective of the present study was the assessment of the effect of noninvasive respiratory support on the SWC of neonates within 28 - 32 weeks of gestation.
4. Results
Table 2 shows the study indicators. The average number of SWCs increased when neonates were supported by nCPAP, which was not statistically significant in comparison to that when neonates were provided with NIPPV support. The mean duration of the SWC when the neonates received nCPAP was significantly longer than when they received NIPPV (P = 0.002). There was a significant increase in the mean duration of the QS cycle (P = 0.000), compared to that of the time neonates received NIPPV. The mean duration of REM sleep in neonates’ SWC exposed to nCPAP was significantly longer (P = 0.035) than when they were exposed to NIPPV.
| Item | nCPAP | NIPPV | P Value |
|---|---|---|---|
| SWC number | 6.44 ± 2.16 | 5.88 ± 1.73 | 0.113 |
| SWC duration (min) | 51.32 ± 11.41 | 48.32 ± 10.04 | 0.002 |
| REM sleep duration (min) | 32.68 ± 5.03 | 21.48 ± 4.71 | 0.035 |
| NonREM sleep duration (min) | 28.04 ± 8.89 | 26.84 ± 7.38 | 0.000 |
| Oxygen saturation (%) | 94.50 ± 2.32 | 93.42 ± 2.47 | 0.021 |
| Mean blood pressure (mmHg) | 51.30 ± 10.21 | 50.63 ± 11.35 | 0.661 |
| Respiratory rate (breath/min) | 58.43 ± 3.18 | 60.33 ± 2.06 | 0.012 |
| Heart rate (beat/min) | 139 ± 15.21 | 140.43 ± 17.02 | 0.538 |
| PCO2 (mmHg) | 44.31 ± 7.66 | 46.22 ± 9.25 | 0.052 |
Abbreviations: nCPAP, nasal continuous positive airway pressure; NIPPV, nasal intermittent positive pressure ventilation; SD, standard deviation; SWC, sleep-wake cycle; REM, rapid eye movement; PCO2, partial pressure of carbon dioxide.
a Values are expressed as mean ± SD.
5. Discussion
In several studies comparing these two medical approaches (nCPAP and NIPPV) to manage respiratory distress, research approaches have been limited to monitoring brain activity, some of which will be discussed in this section. A study was conducted in 2007 by Dani et al. to monitor brain oximetry in neonates involved in RDS under nCPAP intervention. In the aforementioned study, 14 neonates suffering from RDS with less than 30 weeks of gestation received nCPAP respiratory support 12 hours after their extubation with FiO2 ≤ 40%; however, CDP was set at 2 cm H2O for 30 minutes and then at 4 cm H2O for 60 minutes, at 6 cm H2O for another 60 minutes, and finally at 4 cm H2O for 30 minutes. During the whole period, cerebral regional oxygen saturation (crSO2) was monitored. The aforementioned study did not show a significant difference in crSO2 at different levels of CDP (15).
In another study conducted by Lemmers et al., 38 neonates with less than 32 weeks of gestation were monitored by crSO2 for 72 hours after birth. Among these newborns, 18 cases suffered from RDS and underwent conventional mechanical ventilation, and respiratory management was performed in a way that the partial pressure of carbon dioxide was maintained within the range of 40 - 50 mmHg. In the aforementioned study, no significant difference was observed between the two groups in either crSO2 or fractional tissue oxygen extraction (FTOE); however, in the RDS group, at different durations, the records observed for crSO2 and FTOE showed wide and significant variances (17).
In another study conducted by Sadeghnia et al., 30 very low birth weight neonates with RDS underwent nCPAP and NIPPV alternately on the 3rd day of life for 12-hour periods. Although the inhaled oxygen fraction was constant, crSO2 was significantly higher in the nCPAP group (10). Finally, in a study carried out by Elsayed et al., 10 neonates with less than 32 weeks of gestation who had undergone sigh positive airway pressure (SiPAP) and experienced cardiovascular stability were exposed to nCPAP and SiPAP for intermittent 3-hour periods. The aforementioned study did not show a significant difference in neonatal crSO2 during these intermittent periods (21).
The present study showed that, although the average number of SWCs when neonates received nCPAP did not differ significantly from the average number of cycles when they received NIPPV, the average duration of each cycle was significantly longer when neonates received nCPAP, meaning that the average sleep time under nCPAP was longer. Therefore, if this finding is compared to those of the study conducted by Sadeghnia et al. (10), which showed that the mean crSO2 was significantly higher than NIPPV when neonates received nCPAP, it can be concluded that physiological stability in the central nervous system (CNS) was more optimized when neonates received nCPAP.
On the other hand, the duration associated with REM sleep while neonates were under nCPAP was significantly longer than when they were under NIPPV. As it is known, REM is associated with the maximum brain activity during sleep, which means that, at this stage of sleep, endogenous stimuli are formed as reciprocal oscillations between sensory organs on the one hand and the brainstem, thalamus, cortex, the vital process of targeting and axon growth. A clear example is ponto-geniculo-occipital waves, a multitude of which a neonate experiences, which ultimately strengthen learning through the formation of a greater number of connections (22-25).
The duration associated with NonREM sleep was significantly longer when neonates were under nCPAP, compared to the duration when they were under NIPPV. During stages 3 and 4 of NonREM sleep, short-term neurosensory content is transferred from the neocortex to the hippocampus, the amygdala, and the limbic lobe to be retrieved in the structure of complex concepts which is known as memory and cognition. Therefore, the deepening of sleep levels in NonREM sleep, which is undoubtedly accompanied by its prolongation, plays a vital role in the sustainable development of those neural circuits which are essential for learning and memory and maintaining brain flexibility throughout life (26).
5.1. Conclusions
Despite its limitations and delimitations, such as the low sample size, this study demonstrated how different patterns of noninvasive respiratory management could affect the SWC (as shown in Figures 1 and 2). Furthermore, it has been shown that the SWC plays a vital role in the initial building of topographic alignment and organization in the CNS and that any intervention or deprivation of SWC might have a negative, lasting effect on the pruning of the CNS (27). It is recommended to perform a similar study with a larger sample size considering that several studies are being conducted to improve the position of noninvasive respiratory support using two pressure levels in a cyclic and unsynchronized fashion and considering it as standard care.
What is already known on this topic? A similar study showed that the regional saturation of the brain was differently affected when neonates were treated by nCPAP and NIPPV.
What does this study add? The SWC can be differently affected when neonates are treated by nCPAP and NIPPV.