1. Context
It has been more than a year since the official announcement of Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2), leading to Coronavirus disease 2019 (COVID-19) (1). More than a hundred million individuals have already been affected by COVID-19, with more than two million deaths (2). Furthermore, the involvement of multiple organs, including the heart, has been reported by numerous studies (3-8).
Since the pandemic's beginning, researchers have enlightened the world with numerous novel discoveries. Overwhelming evidence shows that COVID-19 children suffer from milder clinical courses than adults (9-11). Nevertheless, Multisystem Inflammatory Syndrome in Children (MIS-C), associated with COVID-19, was such an immense discovery, raising awareness about the potential effects of this disease on children (12). Studies suggest that most cases had a positive history of COVID-19 or exposure to people positive for SARS-CoV-2 in the previous 2 - 4 weeks (13-15). Three well-known guidelines have made efforts to describe this syndrome lately. The World Health Organization (WHO) (16), the Centers for Disease Control and Prevention (CDC) (17), and the Royal College of Pediatrics and Child Health (RCPCH) (18) issued the guidelines to describe this syndrome in May 2020. In this study, the CDC and the WHO criteria were considered, as they include a more eminent COVID-19 element, including either positive real-time reverse transcription-polymerase chain reaction (rRT-PCR), serology, or antigen tests, or prior COVID-19 exposure. These two guidelines include the signs of multisystem organ involvement in children, evidence of inflammation, and the abovementioned COVID-19 requirement with no other presumable diagnosis (19).
Severe MIS-C can result in fatal outcomes, requiring special attention from researchers (14, 20). As multi-organ involvement in this syndrome also affects the heart as a vital organ, there is a need to undertake extensive efforts to minimize this risk (21, 22). Echocardiography is a crucial imaging modality aiding adults and pediatric cardiologists to maximize the provided patient care (23, 24).
Bearing the abovementioned facts in mind, we performed this study to identify and summarize the echocardiographic findings and find their prevalence in the studies of MIS-C associated with COVID-19.
2. Evidence Acquisition
2.1. Design
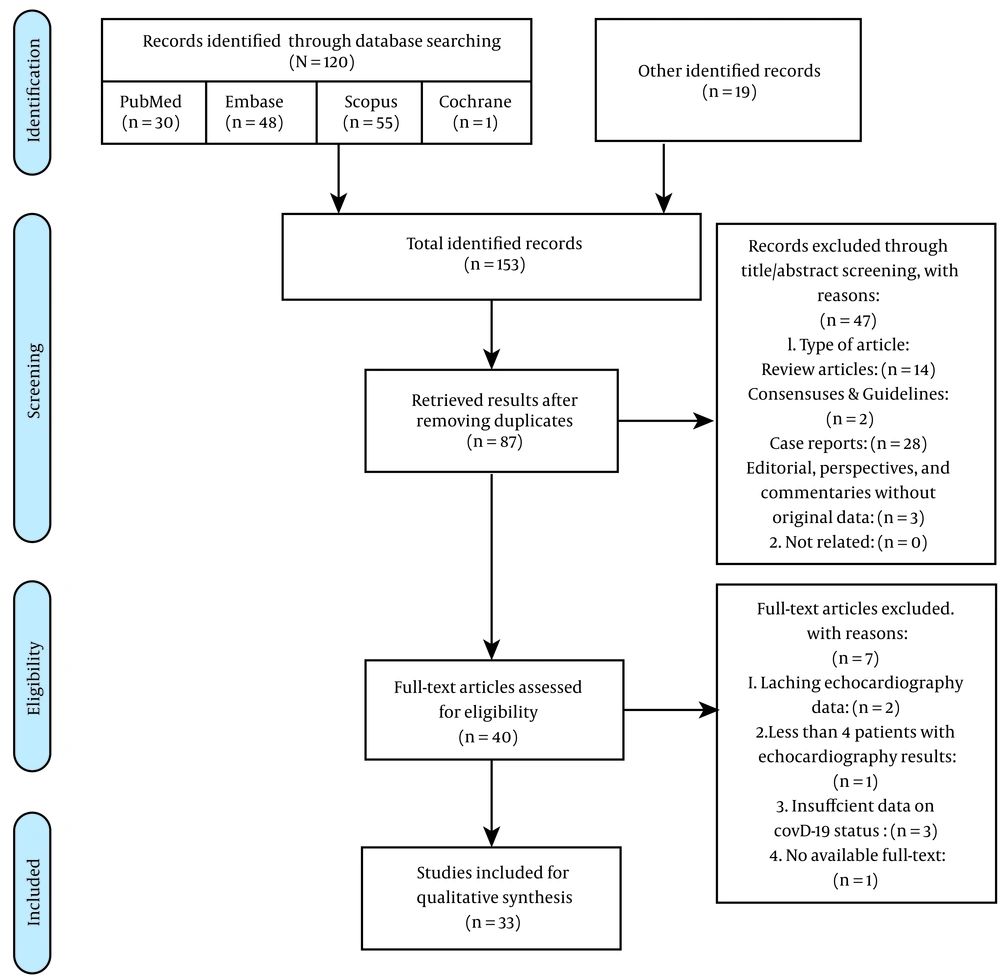
We systematically searched PubMed, Embase, Scopus, and Cochrane databases on December 19, 2020. Upon identifying the studies, two researchers undertook the screening process. Their eligibility was assessed through title/abstract screening at the first step. In this step, we retained the articles, not ascertaining echocardiographic results, to further check them for the presence of this data in the articles' full texts. These identified records further went through the full-text screening process, while relevant articles were included in the qualitative synthesis.
2.2. Search Strategy
We included a title, abstract, and keyword systematic search for various entry terms, including MESH terms, for MIS-C and COVID-19/SARS-CoV-2, combined with an all-field search of echocardiography and reasonable alternatives (Appendix 1 in Supplementary File).
2.3. Full-text Screening Exclusion Criteria
We excluded studies: (1) not reporting any echocardiographic findings; (2) lacking MIS-C criteria, as discussed below; (3) lacking COVID-19 criteria, as discussed below; (4) recruiting adults without specifying the observed findings of children; (5) being abstracts, conference proceedings, and any article without identifiable full-texts; (6) lacking original data, including reviews and non-original editorials; (7) being case reports or articles reporting echocardiographic results for three or fewer patients.
2.3.1. MIS-C Eligibility Criteria
We included the articles that recruited the patients based on the WHO or CDC MIS-C criteria. In the case of articles using any other criteria or not utilizing specific criteria for any reason (e.g., articles reporting the cases before the introduction of these criteria), a researcher checked the full text to identify if the included individuals met the WHO or CDC criteria. As suggested by the CDC (17), we checked the articles that used Kawasaki or atypical Kawasaki disease criteria to determine if they could be included using the abovementioned process.
2.3.2. COVID-19 Eligibility Criteria
Based on the CDC and WHO criteria, patients were considered eligible for COVID-19 criteria if they had either positive RT-PCR, serology, or antigen tests, or prior COVID-19 exposure. When the studies utilized any inclusion criteria (e.g., using RCPCH guideline) not requiring this element, we checked the articles' full texts to identify whether this element was present. Nevertheless, studies remained eligible if they reported the specific results of the COVID-19-eligible subpopulation.
2.4. Recovery of Exclusion Candidates in the Full-text Screening Process
Another researcher rechecked the availability of full texts to find them if possible. Whenever any article lacked the aforementioned COVID-19 eligibility criteria based on the WHO or CDC, we contacted the authors via email to request the eligible patients' data. We included studies with at least around 90% of the patients adhering to the abovementioned MIS-C criteria or COVID-19 eligibility criteria. These measures were taken to minimize the loss of invaluable data.
2.5. Data Acquisition and Prevalence Calculation
A researcher extracted the data, while two others checked the acquired results. To report the age groups, we utilized a classification system used by Feldstein et al. (13) and Swann et al. (25). They categorized the patients into below one year, 1 - 4 years, 5 - 9 years, 10 - 14 years, and above 15 years. However, this classification was not maintained in the studies reporting age groups differently. To calculate the prevalence of an echocardiographic parameter, we divided the number of total observed cases by the total population of the studies reporting that parameter.
2.6. Risk of Bias Assessment
We utilized the Newcastle-Ottawa Scale (NOS) for non-randomized studies to calculate the risk of bias in cohort and case-control studies. A maximum score of 9 could be assigned to each study. The National Institutes of Health (NIH) quality assessment tool (26) was applied to all studies, including case series. We chose 7 - 9, 4 - 6, and 0 - 3 to represent good, fair, and poor ratings for the case series. For cohorts and cross-sectional studies, 11 - 14, 6 - 10, and 0 - 5 were chosen. These numbers were 9 - 12, 5 - 8, and 0 - 4 for case-controls.
3. Results
After searching four databases and adding 19 citations based on the strategy mentioned in section 2.1, 87 non-duplicate studies were included in the screening process. Of them, we found 40 qualified articles in the title/abstract screening and a total of 33 eligible studies for conducting qualitative analysis after the full-text screening process.
Seven articles were excluded from the full-text screening process. Two studies lacked any echocardiographic findings. One of the studies only reported three patients undergoing echocardiography. Three studies recruited patients without desirable COVID-19 criteria mentioned in section 2.3.2. We also contacted five articles' corresponding authors to request further clarifying data on their patients’ COVID-19 status; two of them were already included based on the percentage rule discussed in section 2.3.2; unfortunately, none was with a positive response. Furthermore, we could not find a study's full text based on the conditions mentioned in section 2.4 (Figure 1).
Finally, the included studies recruited a total of 1,392 patients with MIS-C. Girls contributed to 601 (43.2%) and boys to 791 (56.8%) of the population. The mean age of the patients was 8.3 ± 5.9 years. Fourteen studies with 426 patients had available age groups data, as mentioned in section 2.5. Of these patients, 19 (4.5%) were under one year, 86 (20.2%) were 1 - 4 years, 131 (30.8%) were 5 - 9 years, 131 (30.8%) were 10 - 14 years, and 59 (13.8%) were above 15 years, making children under one year the least frequent, and 5 - 9 and 10 - 14 years old the most prevalent groups (Table 1).
| ID | Study | Study Design | Total pop. | Female; No. (%) | Age Groups a | Underlying Conditions | Summary of Echocardiographic Findings |
|---|---|---|---|---|---|---|---|
| 1 | Abdel-Mannan et al. (27) | Case series | 4 | 2 (50%) | 11.7 ± 3.77; < 1 y: 0 (0%); 1 - 4 y: 0 (0%); 5 - 9 y: 2 (50%); 10 - 14 y: 0 (0%); 15 ≤ y: 2 (50%) | Obesity, 1 (25%) | Normal, n = 2 (50%); Mild pericardial effusion, n = 1 (25%); Mitral regurgitation, n = 1 (25%); Mild to moderate ventricular impairment; n = 1 (25%); No coronary arteriopathy |
| 2 | Belhadjer et al. (28) | Case series | 35 | 17 (49%) | 9.2 ± 10.8; < 1 y: 0 (0%); 1 - 5 y: 1 (2.9%); 6 - 10 y: 15 (42.9%); 11 - 16 y: 19; (54.3%) | Total, 10 (28%): Asthma, 3 (8.5%); Lupus, 1 (3%); Overweight, 6 (17%) | Coronary artery dilatation Z score > 2, n = 6 (17%); Aneurysms: 0 (0); LVEF at baseline; < 30%, n = 10 (28%); 30% - 50%, n = 25 (72%); Evolution of LVEF, median ± SD, %; Baseline (35 patients): 32 ± 9; Day 3 (23 patients): 52 ± 10; Day 7 (34 patients): 60.6 ± 6; Left ventricular hypokinesis, n = 31 (88%); Segmental wall hypokinesis, n = 3 (8%); Takotsubo syndrome presentation with akinesis of the apical segment, n = 1 (3%); Right ventricular dysfunction, n = 0; Pericardial effusion, n = 3 (8%) |
| 3 | Biko et al. (29) | Retrospective cohort | 10 | 6 (66.7%) | 9.66 ± 3.8; < 1 y: 0 (0%); 1 - 4 y: 0 (0%); 5 - 9 y: 6 (60%); 10 - 14 y: 3 (30%); 15 ≤ y: 1 (10%) | Total, 3 (30%): neurologic disease; asthma; prematurity | Nine patients underwent echocardiography; LVEF (%) mean (range): 55.0 (42.0 to 58.0); Abnormality threshold values: > 55; LVSF (%) mean (range): 30.0 (24.0 to 34.0); Abnormality threshold values: > 25; GLS (%) mean (range): -15.5 ( -18.0 to - 12.5); Abnormality threshold values: > -20.2; TAPSE (cm) mean (range): 1.9 (1.4 to 2.0); Abnormality threshold values: > 1.7; RVFWS (%) mean (range): -15.6 (-19.8 to -13.6); Abnormality threshold values: > -27.2 |
| 4 | Blondiaux et al. (30) | Case series | 4 | 3 (75%) | 9.25 ± 2.75; < 1 y: 0 (0%); 1 - 4 y: 0 (0%); 5 - 9 y: 2 (50%); 10 - 14 y: 2 (50%); 15 ≤ y: 0 (0%) | N/A | Appearance of the myocardium: normal (n = 3); Diffuse echo-bright appearance, (n = 1); Echogenic myocardium on 2D echocardiography, n = 2 (50%); LVEF < 30%, n = 1 (25%); Low-normal LVEF > 50%, n = 3 (75%); Functional Mitral regurgitation due to LV dilation, n = 2; Moderate pericarditis, n = 3; Dyskinesis/hypokinesis: hypokinesis (n = 2), septal dyskinesis (n = 1), none (n = 1); LVEF (%): 24, 61, 54, 59; LVSF (%): 10, 33, 30, 31; VTI (cm): 10, /, 15, 16.5; HR (bpm): 147, /, 162, 124; Pericardial effusion, n = 2 of 4; Coronary artery; Dilatation/aneurysm, n = 0; In follow-up echocardiograms, no abnormality was observed. |
| 5 | Blumfield et al. (31) | Retrospective cohort | 16 | 6 (38%) | 9.2 ± 4.9; Age groups: N/A | Total, n = 9 (56%); Obesity, n = 4 (25%); Asthma, n = 3 (19%); Sickle Cell Disease, n = 1 (6%); Small VSD, n = 1 (6%); Recurrent UTIs, n = 1 (6%) | Echocardiography abnormalities, n = 13 (81%); Systolic myocardial dysfunction, n = 10 (63%); Coronary artery ectasia, n = 4 (25%); Pericardial effusion, n = 2 (13%); Cardiac injury due to echo, n = 66%; Coronary aneurysms, n = 0 |
| 6 | Caro-Paton et al. (32) | Case series | 12 | 4 (33%) | 9.6 ± 2.7; < 1 y: 0 (0%); 1 - 4 y: 0 (0%); 5 - 9 y: 6 (50%); 10 - 14 y: 6 (50%); 15 ≤ y: 0 (0%) | N/A | Echocardiography was performed on 11 patients; Mildly decreased LVEF, n = 4; Dilated LA and coronary artery ectasia, n = 1 |
| 7 | Carter et al. (33) | Prospective cohort | 17 | 4 (24%) | 13.3 ± 3.4; < 5 y: 1 (6%); 5 ≤ y: 16 (94%) | 2 (12%) | Coronary artery aneurysms, n = 7 (41%); Worst coronary artery Z score: 1.77 (1.94); Worst left ventricular fractional shortening: 24.2 (4.8) |
| 8 | Cheung et al. (34) | Case series | 17 | 9 (53%) | 8.2 ± 3.9; Age groups: N/A | N/A | LV function; Normal, n = 6 (35%); Mildly decreased, n = 5 (29%); Mild-moderately decreased, n = 4 (24%); Moderate-severely decreased, n = 2 (12%); Pericardial effusion, n = 8 (47%); prominent or echogenic coronary arteries, n = 7; medium-sized; coronary aneurysm (Z score, 5.2), n = 1 |
| 9 | Clark et al. (22) | Retrospective cohort | 55 | 21 (38%) | 7 ± 5.2; < 1 y: 4/52 (7.7%); 1 - 4 y: 17/52 (32.7%); 5 - 9 y: 15/52 (28.8%); 10 - 14 y: 12/52 (23.1%); 15 ≤ y: 4/52 (7.7%) | None | Decreased LV function (LVEF < 60%), n = 35 (64%); Mildly decreased (51 < EF < 60%), n = 18 (33%); Moderately-decreased (41 < EF < 50%), n = 11 (20%); Severely-decreased (EF < 40%), n = 6 (11%); Valvulitis, n = 17 (31%); Pericardial effusion, n = 12 (22%); Coronary abnormalities, n = 11 (20%); (Brightness without dilation by Z score, n = 2 (18%); Coronary dilation, n = 9 (82%); Coronary aneurysms, n = 1 (9%); Additional echocardiographic findings in patients with coronary abnormalities; Pericardial effusion, (n = 5); Mitral regurgitation, (n = 3); Aortic insufficiency, (n = 1); Tricuspid regurgitation, (n = 1); LV dilation, (n = 1) |
| 10 | Corwin et al. (35) | Retrospective cohort | 33 | 18 (54.5%) | 7 ± 8.5; Age groups: N/A | None | 18 patients underwent echocardiography; Abnormal ventricular function, n = 3; Coronary artery dilation, n = 2 |
| 11 | de Farias et al. (36) | Case series | 11 | 2 (18%) | 6 ± 3.4; < 1 y: 1 (9.1%); 1 - 4 y: 5 (45.5%); 5 - 9 y: 3 (27.3%); 10 - 14 y: 2 (18.2%); 15 ≤ y: 0 (0%) | Severe poor Nutrition, n = 2; overweight, n = 3; obese, n = 3 | Abnormal echocardiography, n = 7 (63%); Mild aneurysms(n = 4), medium aneurysm (n = 3); EF = 30%, n = 1; EF ≥ 60%, n = 10; Slight tricuspid and pulmonary reflux, n = 2; Pericardial stroke, n = 6; Left chambers dilation, n = 2; Mild Mitral regurgitation, n = 1 |
| 12 | Dhanalakshmi et al. (37) | Case series | 11 | 7 (64%) | 9.4 ± 11.7; Age groups: N/A | n = 1 (9%) | Coronary artery changes, n = 1 of 11 (9%); dilatation without aneurysms (Z score < 2.5 and evidence of minimal pericardial effusion) |
| 13 | Feldstein et al. (13) | Prospective and retrospective cohort | 186 | 73 (39%) | 8.0 ± 6.9; < 1 y: 13 (7%); 1 - 4 y: 53 (28%); 5 - 9 y: 46 (25%); 10 - 14 y: 45 (24%); 15 ≤ y: 29 (16%) | Patients with at least one comorbidity excluding obesity; n = 51 (27%); Respiratory, n = 33 (18%); Cardiac, n = 5 (3%); Immune system; n = 10 (5%); Other systems; n = 20 (11%); BMI-based obesity; n = 45 (29%) | Patients with at least one echocardiogram; n = 170 (91%); Decreased LV Function, n = 70 (38%); 35% < EF < 55%, n = 61 (33%); 35% > EF, n = 9 (5%); Mitral valve regurgitation (n = 57, 34%); Pericarditis or pericardial effusion (n = 49, 29%); LAD or RCA Z score ≥ 2.5, n = 15 (9%) |
| 14 | Gaitonde et al. (38) | Retrospective cohort | 12 | 3 (25%) | 8.4 ± 5; Age groups: N/A | None | Tricuspid regurgitation: Less than mild n = 8 (67%); Mild or greater n = 4 (33%); Mitral regurgitation: Less than mild n = 6 (50%); Mild or greater n = 6 (50%); Pericardial effusion, n = 5 (42%); Coronary arteries: Coronary artery aneurysm, n = 0; LMCA Z score: ≤ 2, n = 11 (92%); > 2, n = 1 (8%); LAD Z score: ≤ 2, n = 12 (100%); > 2, n = 0; LCX Z score: ≤ 2, n = 8 (100%) |
| 15 | Godfred-Cato et al. (14) | Case series | 570 | 254 (44.6%) | 8 ± 5.9; Age groups: N/A | Obesity, n = 146 (25.6%); Chronic lung disease, n = 48 (8.4%) | Echocardiography was performed on 510 patients; Cardiac dysfunction, n = 207 (40.6%); Coronary artery dilatation or aneurysm, n = 95 (18.6%); Pericardial effusion, n = 122 (23.9%); Mitral regurgitation, n = 130 (25.5%) |
| 16 | Grimaud et al. (39) | Case series | 19 | 9 (47%) | 8.9 ± 3.2; < 1 y: 0 (0%); 1 - 4 y: 3 (15.8%); 5 - 9 y: 8 (42.1%); 10 - 14 y: 7 (36.8%); 15 ≤ y: 1 (5.3%) | N/A | Left ventricular ejection fraction; mean (SD) = 36.4% (8.7); Decreased LV function (EF < 60), n = 19; Mildly decreased (50 < EF ≤ 59%), n = 1 (5%); Moderately-decreased (40 < EF ≤ 50%), n = 5 (26%); Severely-decreased (EF ≤ 40%), n = 13 (68%); Pericardial effusion, n = 4; Coronary artery dilation or aneurysms, n = 0 |
| 17 | Jain et al. (40) | Case series | 23 | 12 (52%) | 7.5 ± 2.9; Age groups: N/A | N/A | LV systolic dysfunction, n = 8 (34.8%); Coronary dilation, n = 6 (26%) |
| 18 | Jhaveri et al. (41) | Retrospective cohort | 15 | 6 (40%) | 11.5 ± 4.49; Age groups: N/A | N/A | Average LVEF: 49.5% (range, 29 - 58%); LV dysfunction (EF < 55%), n = 8; 50% < EF < 54% (n = 2); 40% < EF < 49% (n = 4); EF < 35% (n = 2); RV dysfunction, n = 4 (27%); Small pericardial effusion, n = 2; Mitral regurgitation, n = 8(53%); Coronary artery involvement (Z score > 2.5); n = 4 of 12 (33%); Aneurysm in LCA (n = 2), RCA (n = 1), LADA (n = 3) |
| 19 | Lee et al. (42) | Retrospective cohort | 28 | 12 (43%) | 8.9 ± 4.2; Age groups: N/A | Total (n = 14), Obesity (n = 4); Asthma (n = 3); Congenital heart disease (n = 1); Sickle cell anemia (n = 1); Mitochondrial disorder (n = 1); Autism (n = 1); Chromosomal abnormalities (n = 1) | LV dysfunction (EF < 55%), n = 11 (39%); EF < 30%, n = 2; Dilated coronary vessel, n = 2 (7%); Coronary aneurysm, n = 4 (14%) |
| 20 | Mamishi et al. (43) | Case series | 45 | 21 (47%) | 7.3 ± 4.5; Age groups: N/A | Total, 6 (13%); ALL, CKD, Underlying seizure disorder, CP, CVD, and Budd-Chiari syndrome | Cardiomegaly, n = 2 (4%); Coronary dilation, n = 14 (31%); Pericardial effusion, n = 1 (2%); Myocarditis, n = 8 (18%) |
| 21 | Matsubara et al. (44) | Retrospective cohort | 28 | 14 (50%) | 11 ± 4.5; < 1 y: 0 (0%); 1 - 4 y: 2 (7.1%); 5 - 9 y: 9 (32.1%); 10 - 14 y: 12 (42.9%); 15 ≤ y: 5 (17.9%) | None | MR > trivial, n = 13 (46%); Pericardial effusion, n = 9 (32%); Pleural effusion, n = 11 (39%); LVEF, % 57 (48 - 61); Coronary dilatation n = 0 (0); Coronary ectasia, n = 1 (4%); Coronary aneurysm, n = 0 (0) |
| 22 | Minocha et al. (21) | Retrospective cohort | 33 | 14 (42%) | 4.5 ± 5.8; Age groups: N/A | Total, n = 12 (36%); Obesity, n = 7 (21%); Asthma, n = 5 (15%) | Echocardiography was performed on 30 patients; Abnormal echocardiogram, n = 10 (33%); Left ventricular dysfunction n = 4 (13%); Median EF = 45% (IQR: 44% to 48%); Non-physiologic Mitral regurgitation n = 4(13%); LCMA dilation, n = 2 (7%); Regional wall motion abnormalities, n = 1 (3%); Pericardial effusion, n = 1 (3%) |
| 23 | Perez-Toledo et al. (45) | Retrospective cohort | 8 | 3 (37%) | 9.6 ± 2.4; Age groups: N/A | N/A | Five (63%) of patients had impaired myocardial function. |
| 24 | Pouletty et al. (46) | Retrospective cohort | 16 | 8 (50%) | 9 ± 6.3; Age groups: N/A | Total, n = 6 (37%); Asthma, n = 2; Overweight, n = 4 | Echocardiographic abnormalities, n = 11/16 (69%); Coronary dilation, n = 3 (19%); Myocarditis, n = 7 (44%); Pericarditis, n = 4 (25%) |
| 25 | Ramcharan et al. (47) | Case series | 15 | 4 (27%) | 8.8 ± 3.9; < 5 y: 1 (7%); 5 ≤ y: 14 (93%) | N/A | Coronary artery abnormalities, n = 14 (93%); Moderate fusiform aneurysm of the right coronary (n = 1); Ectatic dilated coronaries with increased Z scores (n = 6); Prominent coronary arteries, (n = 7); Atrioventricular valve regurgitation, (n = 13); Mitral regurgitation, (n = 10); Non-physiological tricuspid regurgitation (n = 7); Decreased ventricular Function Fractional Shortening, n = 8 (Median = 29%); Impaired LVEF, n = 12 (80%); Severe (EF < 30%), n = 1; Moderate (30% < EF < 45%), n = 3; Mild (45% < EF < 55%), n = 8; MAPSE (Z score < -2), n = 11; TAPSE (Z score < -2), n = 10; Diastolic dysfunction (n = 2); Small pericardial effusion (n = 8) |
| 26 | Riollano-Cruz et al. (48) | Case series | 15 | 4 (27%) | 12.1 ± 2.8; < 1 y: 0 (0%); 1 - 4 y: 1 (6.7%); 5 - 9 y: 3 (20%); 10 - 14 y: 7 (46.7%); 15 ≤ y: 4 (26.7%) | Total, n = 5 (33%); Asthma, (n = 4); Hypothyroidism and NAFLD (n = 1) | Echocardiography was performed on 14 patients; Decreased LV systolic function, n = 7 (low-normal = 1; mild = 2; moderate = 2, severe = 2); Decreased RV function, n = 3 (mild = 1; moderate = 1; severe = 1); Pericardial effusions (trivial to small), n = 7; Mitral regurgitation (trivial to mild), n = 6; Tricuspid regurgitation, n = 8 (mild = 6; moderate = 2); Coronary artery abnormalities: Dilated (n = 1); ectatic (n = 3); prominent (n = 1); aneurysm (n = 0) |
| 27 | Riphagen et al. (49) | Case series | 5 | 2 (40%) | 8.4 ± 2.6; < 1 y: 0 (0%); 1 - 4 y: 0 (0%); 5 - 9 y: 4 (80%); 10 - 14 y: 1 (20%); 15 ≤ y: 0 (0%) | n = 1 (20%); Autism and ADHD | Mild to moderate LV systolic dysfunction (n = 2); RV dysfunction (n = 1); Biventricular dysfunction (n = 1); Severely dilated coronary arteries (n = 1); Peri-coronary hyper-echogenicity (n = 1); AV valve regurgitation (n = 1) |
| 28 | Sadiq et al. (50) | Case series | 8 | 1 (12.5%) | 9.5 ± 1.3; < 1 y: 0 (0%); 1 - 4 y: 0 (0%); 5 - 9 y: 4 (50%); 10 - 14 y: 4 (50%); 15 ≤ y: 0 (0%) | None | LV systolic dysfunction (EF < 55%), n = 2 (25%); Pericardial effusion (small), n = 4 (50%); Coronary abnormalities, n = 5 (62.5%) |
| 29 | Shobhavat et al. (51) | Case series | 21 | 11 (52%) | 7.2 ± 3.8; Age groups: N/A | Aplastic anemia, n = 1 (5%) | Decreased LV systolic function, n = 9 (43%); 30% < EF < 55%, n = 7 (33%); EF < 30%, n = 2; Coronary dilation (Z score > 2.5), n = 5 (24%); |
| 30 | Swann et al. (25) | Prospective Cohort | 52 | 21 (40.4%) | 11 ± 4.4; < 1 y: 1 (1.9%); 1 - 4 y: 4 (7.7%); 5 - 9 y: 16 (30.8%); 10 - 14 y: 22 (42.3%); 15 ≤ y: 9 (17.3%) | Total, n = 15 (28.8%); Obesity, n = 5 (9.6%) | Echocardiography was performed on 37 patients; Impaired cardiac function on echocardiogram, n = 10; Pericardial effusion, n = 9; Coronary artery dilatation, n = 3; Coronary artery aneurysm, n = 2; Myocarditis, n = 2; Valvular regurgitation, n = 2 |
| 31 | Theocharis et al. (52) | Retrospective cohort | 20 | 5 (25%) | 10.6 ± 3.8; < 1 y: 0 (0%); 1 - 4 y: 1 (5%); 5 - 9 y: 7 (35%); 10 - 14 y: 8 (40%); 15 ≤ y: 4 (20%) | N/A | EF < 55%, n = 10 (50%); Valvular regurgitation, n = 15 (75%); Small pericardial effusions, n = 2 (10%); Increased pericardial brightness, n = 20 (100%); Increased echogenicity of the interventricular septum, n = 5 (25%); LV systolic dysfunction, n = 10 (50%); LV systolic performance, assessed by TDI and strain echocardiography was reduced in 18 (90%) patients; Mean GLS = -13.2±2.9; E/e' > 8 as evidence of increased LV filling pressure, n = 9 (45%); Mitral regurgitation; Mild, n = 10 (50%); Moderate to severe, n = 2 (10%); Tricuspid regurgitation; Mild, n = 12 (60%); Moderate to severe, n = 3 (15%); Trivial AR, n = 1 (5%); Coronary artery dilation, n = 3 (15%) |
| 32 | Torres et al. (53) | Case series | 27 | 13 (48%) | 6.25 ± 3.5; Age groups: N/A | 20 (74%) | Echocardiography was performed on 26 patients; Abnormal echo finding on admission, n = 8 (31%); throughout hospitalization: n = 12; Myocardial dysfunction on admission, n = 4 (15%); throughout hospitalization: n = 4; Coronary abnormalities, n = 3 (%12); throughout hospitalization: n = 5; Myocardial and coronary abnormalities, n = 1 (4%); Pericardial effusion, n = 3 (11%) (developed throughout the hospitalization) |
| 33 | Toubiana et al. (54) | Case series | 21 | 12 (57%) | 8.5 ± 3.4; Age groups: N/A | None | Coronary abnormalities, n = 8 (38%); Increased visibility, n = 3 (14%); Dilation (Z score 2 to < 2.5), n = 5 (24%); Aneurysm (Z score ≥ 2.5), n = 0 |
Details of the Included Studies
Some eligible patients in the studies did not undergo echocardiography, but 1,279 patients had echocardiography reports (113 patients without reports). Comorbidities were present in 359 out of 1,259 patients, accounting for 28.5% of their total population. This comorbidity comparison did not include studies not reporting comorbidities or intentionally recruiting previously healthy children. Overweight/obese and chronic lung disease, including asthma, were the most commonly reported underlying conditions (details in Table 1). The studies were conducted in nine countries from Asia, Europe, North America, and South America. Fourteen studies were classified as cohorts, and the rest (n = 17) was case series. Table 1 illustrates a comprehensive report of the included studies.
We divided the conventional echocardiographic findings of acute-phase MIS-C into four main categories: Ventricular, coronary, valvular, and pericardial involvement. In the case of ventricular involvement, all of our 33 studies reported data about left ventricular (LV) systolic dysfunction, and of 1,392 patients, 486 (34.91%) had an LV ejection fraction (LVEF) < 55. However, in conventional echocardiographic findings, LV diastolic dysfunction and right ventricular (RV) dysfunction were under-appreciated. In the case of coronary involvement, including coronary arteries ectasia, dilation, aneurysm, and other miscellaneous entities, available data from 29 studies with 1,356 patients showed that 245 (18%) had coronary involvement. Besides, 13 studies with 1,052 patients reported data about valvular involvement. Apart from two cases of aortic insufficiency, other reported valvular involvement were Mitral regurgitation (MR) and tricuspid regurgitation (TR). Also, 306 (29.08%) patients had valvular involvement, of whom 259 (24.61%) had MR, and 45 (4.28%) had TR. Data about pericardial involvement, including pericarditis and pericardial effusion, were available in 19 studies with 1,116 patients, of whom 252 (22.58%) showed evidence of pericardial involvement.
Table 2 presents the detailed NOS scores attributed to the cohorts. The mean [standard deviation (SD)] score was 6.7 (0.5) out of nine. The NIH quality assessment score of all the included studies is illustrated in Table 3. Our ratings based on the scores categorized 16 case series into good quality studies, and two others had fair quality. We recognized eight cohort studies in the good category and seven articles in the fair category.
| ID | Author | Reference | Selection | Comparability | Exposure | Total Score |
|---|---|---|---|---|---|---|
| 3 | Biko et al. | (29) | **** | - | *** | 7 |
| 5 | Blumfield et al. | (31) | **** | - | *** | 7 |
| 7 | Carter et al. | (33) | **** | * | *** | 8 |
| 9 | Clark et al. | (22) | **** | - | ** | 6 |
| 10 | Corwin et al. | (35) | *** | ** | *** | 8 |
| 13 | Feldstein et al. | (13) | **** | - | *** | 7 |
| 14 | Gaitonde et al. | (38) | *** | ** | *** | 8 |
| 18 | Jhaveri et al. | (41) | **** | - | *** | 7 |
| 19 | Lee et al. | (42) | *** | - | *** | 6 |
| 21 | Matsubara et al. | (44) | *** | * | * | 5 |
| 22 | Minocha et al. | (21) | **** | - | *** | 7 |
| 23 | Perez-Toledo et al. | (45) | *** | - | ** | 5 |
| 24 | Pouletty et al. | (46) | *** | - | *** | 6 |
| 30 | Swann et al. | (25) | **** | - | *** | 7 |
| 31 | Theocharis et al. | (52) | *** | - | *** | 6 |
Newcastle-Ottawa Scale Risk of Bias Assessment of the Included Cohorts
| Study | Reference | Total Score | Quality Rating (Good, Fair, or Poor) |
|---|---|---|---|
| Case Series (Score Out of 9) | |||
| Abdel-Mannan et al. | (27) | 7 | Good |
| Blondiaux et al. | (30) | 7 | Good |
| Cheung et al. | (34) | 7 | Good |
| Dhanalakshmi et al. | (37) | 6 | Fair |
| Grimaud et al. | (39) | 6 | Fair |
| Mamishi et al. | (43) | 7 | Good |
| Riollano-Cruz et al. | (48) | 7 | Good |
| Sadiq et al. | (50) | 7 | Good |
| Torres et al. | (53) | 8 | Good |
| Belhadjer et al. | (28) | 8 | Good |
| Caro-Paton et al. | (32) | 8 | Good |
| de Farias et al. | (36) | 7 | Good |
| Godfred-Cato et al. | (14) | 7 | Good |
| Jain et al. | (40) | 8 | Good |
| Ramcharan et al. | (47) | 8 | Good |
| Riphagen et al. | (49) | 8 | Good |
| Shobhavat et al. | (51) | 7 | Good |
| Toubiana et al. | (54) | 9 | Good |
| Cohorts (Score Out of 14) | |||
| Biko et al. | (29) | 11 | Good |
| Carter et al. | (33) | 12 | Good |
| Corwin et al. | (35) | 11 | Good |
| Gaitonde et al. | (38) | 12 | Good |
| Lee et al. | (42) | 9 | Fair |
| Minocha et al. | (21) | 10 | Fair |
| Pouletty et al. | (46) | 10 | Fair |
| Theocharis et al. | (52) | 11 | Good |
| Blumfield et al. | (31) | 11 | Good |
| Clark et al. | (22) | 10 | Fair |
| Feldstein et al. | (13) | 10 | Fair |
| Jhaveri et al. | (41) | 11 | Good |
| Matsubara et al. | (44) | 9 | Fair |
| Perez-Toledo et al. | (45) | 8 | Fair |
| Swann et al. | (25) | 11 | Good |
Applying the National Institutes of Health Quality Assessment Tool for All the Included Studies
4. Discussion
As known, MIS-C is a rare complication of COVID-19 in pediatrics (25) who have a positive recent history of COVID-19 during 1 - 2 weeks before MIS-C manifestations (13). It has a wide range of involvement, such as gastrointestinal, cardiovascular, mucocutaneous, renal, and hematologic manifestations (16-18). Studies show that cardiovascular involvement, such as hypotension and cardiac dysfunction, needs emergent support (13, 14, 39, 42). The high rate of cardiac involvement in patients with MIS-C demonstrates the necessity for early cardiology consultation and follow-up during hospital admission.
Echocardiography is a widely available diagnostic method that provides the operator with reliable and real-time information about the anatomy and function of the heart. Therefore, it has become an irreplaceable tool in the pediatric and neonatal intensive care unit (NICU) to evaluate cardiovascular diseases (55). Here, we categorize the echocardiographic findings based on the anatomic parts and discuss them in detail.
4.1. Ventricular Involvement
One of the cornerstones of cardiovascular characteristics of MIS-C is impaired myocardial function. The pathophysiologic mechanism behind myocardial dysfunction is not apparent to the researchers yet, but some probable explanations exist. Based on their observation, Matsubara et al. suggested subclinical myocarditis as the main reason (44). In the absence of cardiac magnetic resonance (CMR) imaging, high blood levels of NT-pro-BNP and troponin I can support this hypothesis. Conversely, Belhadjer et al. argued that the rapid resolution of systolic dysfunction in most patients and mild to moderate elevations of cardiac biomarkers point to a mechanism other than the myocardial injury caused by direct viral invasion (28). Also, CMR studies in MIS-C patients showed signs of myocardial edema and hyperemia with no evidence of myocardial scarring and necrosis, which is against the direct viral invasion of the myocardium (30, 52).
Several studies reported a decreased LVEF (LVEF < 55%) in their cases, ranging from 9% (36) to 100% (28). The frequency of LV systolic dysfunction was 34.91% (486/1392) among all the included studies. Also, it is critical to understand the level of ventricular impairment in each individual. Belhadjer et al. (28) reported 35 patients with decreased LVEF (baseline LVEF 32 ± 9%) on admission, with 10 (28%) patients having severely impaired LVEF (LVEF < 30%). In a study of MIS-C in the United States, 70 (38%) patients had decreased LVEF, and nine had severe LV systolic dysfunction (13). Clark et al. had a higher cutoff for LV systolic impairment and suggested LVEF < 60%. In their study, 35 (64%) patients had low LVEF, while 18 (33%) patients had mildly decreased (51 < EF < 60%), 11 (20%) patients had moderately decreased (41 < EF < 50%), and six patients had severely decreased LVEF (EF < 40%) (22). Other studies used different methods for reporting LVEF that withheld us from understanding the exact number of patients with reduced LVEF. For example, Biko et al. (29) reported the mean LVEF of 55%, in a range of 42.0 to 58.0%. Some studies also reported the total number of patients with LV systolic dysfunction and did not provide comprehensive information about the extent of ventricular impairment in each individual (14, 25, 45). Such discrepancies in the method of reporting LVEF make it difficult to determine the exact frequency and extent of LV systolic dysfunction among patients. Therefore, reduced LVEF is a common echocardiographic finding in patients with MIS-C, while they have mild levels (45% < EF < 55%) of LV systolic dysfunction in most cases.
More details of LV systolic function and strain patterns from 2-dimensional Speckle Tracking Echocardiography (2D-STE) and Tissue Doppler Imaging (TDI) can support systolic impairment in MIS-C patients. Previous studies in patients with CMR-proven myocarditis, in the setting of viral diseases, suggested that deformity indices can point to ventricular dysfunction, even in patients with preserved LVEF (56). Compared to a normal control group, people with MIS-C had significantly reduced median Global Longitudinal Strain (GLS) (-16.2% vs. -22.5%, P < 0.001) and Global Circumferential Strain (GCS) (-18.1% vs. -23.9%, P < 0.001). Reduced GLS and GCS also exist in a subgroup of MIS-C patients with preserved LVEF compared to normal controls (-18.7% vs. -22.5%, P = 0.005, and -19.8% vs. -23.9%, P < 0.001, respectively). During the early follow-up, LVEF returned to the normal range in most MIS-C patients. The GLS values also improved significantly (median: from -15.5% to -18.7%, P < 0.001) (44). However, it remained less than the normal reference range in pediatrics (57).
Studies of Gaitonde et al. (38) (median: from -14.0% to -19.3%, P = 0.02) and Theocharis et al. (52) (mean ± SD: from -13.2 ± 2.9% to -17.0 ± 2.9%, P = 0.018) demonstrated that a severely reduced GLS on admission improves during the early follow-up period, but remains slightly less than the reference range by hospital discharge, even though LVEF was within the normal range in most of the patients. Also, alongside GLS, Theocharis et al. reported a significant improvement in septal and lateral systolic (s'lat and s'sep) TDI velocities from admission to hospital discharge, but early and late diastolic velocities did not change (52). Lower than normal GLS values by the time of hospital discharge suggest the need for further studies with longer follow-up periods to determine whether the subclinical myocarditis-like pattern persists or not.
Left ventricular diastolic dysfunction was either under-reported or rare; in our systematic review, only one study exclusively reported diastolic dysfunction in two patients on conventional echocardiography (47). Based on TDI echocardiography, Theocharis et al. stated that nine out of 20 enrolled patients with MIS-C had an E/e' ratio < 8 (mean ± SD: 8.3 ± 2.1 on admission), as evidence of increased LV filling pressure. However, these findings were not in line with blood NT-pro-BNP levels to approve a diagnosis of diastolic dysfunction. Also, LV diastolic function assessed by E/A and E/e' ratios did not change significantly from admission to hospital discharge (52). In another study, patients with MIS-C had significantly reduced E/e' ratio and lateral mitral e' velocity on admission, compared to a normal control group. Furthermore, diastolic parameters in 2D-STE, including longitudinal and circumferential early diastolic strain rate and left atrial strain, were significantly lower in the MIS-C group. Diastolic dysfunction persisted in the early follow-up assessments (44). This can be an interesting finding which deserves attention in future studies.
Data about RV function is limited because of under-reporting or its infrequency. Nevertheless, in three studies that reported RV dysfunction (41, 48, 49), eight out of 35 patients had RV dysfunction on conventional echocardiography. In functional studies, right ventricular parameters, i.e., tricuspid annular plane systolic excursion (TAPSE) on TDI and right ventricular free wall longitudinal strain (RVFWLS) on 2D-STE, were significantly decreased in the patients. Matsubara et al. reported a strong association between RVFWLS and myocardial injury in MIS-C [odds ratio (95% CI): 1.59 (1.09 to 2.34)]. Moreover, they reported that TAPSE improved and returned to the normal range. Although RVFWLS improved significantly, it was still below normal, indicating residual RV injury (44).
These findings suggest that the study of myocardial dysfunction by 2D-STE and TDI can provide invaluable data about subclinical myocardial involvement in patients with MIS-C. However, there are three main drawbacks to the application of 2D-STE in the evaluation of patients. First, software-vendor inter-variability makes it hard to define a normal range for echocardiographic strain and strain-rate imaging variables. Second, the impact of heart rate on strain imaging is more evident in pediatrics than in adults, which interferes with the clinical usefulness of results. Therefore, heart rate at rest is an essential factor in interpreting myocardial deformation in children (58). Third, to minimize contact exposure, a comprehensive echocardiographic evaluation may not be achieved in most patients during the COVID-19 pandemic, and thus, it may not be practical in emergent settings.
4.2. Coronary Involvement
One of the challenging issues of MIS-C is its similarity with Kawasaki disease in terms of coronary artery involvement (59). The exact mechanism for developing coronary involvement is uncertain. However, fever and high blood levels of inflammatory mediators in MIS-C may have a prominent role, rather than coronary arterial wall disruption seen in Kawasaki disease. Furthermore, febrile children are more likely to have larger coronary dimensions than afebrile controls (60). Conversely, Whittaker et al. showed no significant difference in inflammatory biomarkers between MIS-C patients who developed coronary artery involvement and patients without coronary involvement. Thus, they assumed that the coronary changes are not entirely related to the hyperinflammatory state in MIS-C patients (61).
To unify the reporting system of coronary dilation in children, coronary artery dimensions are normalized to the body surface area as Z scores based on the normative data obtained from afebrile children. In 2017, the American Heart Association (AHA) defined coronary artery dilation as a Z score of ≥ 2 and > 2.5 and Coronary Artery Aneurysm (CAA) as a Z score of ≥ 2.5 in Kawasaki disease (62). Of 33 included studies, 29 reported coronary artery abnormalities in our literature review. Eighteen percent (245/1356) of cases had coronary artery abnormality; among them, 78 patients had coronary artery dilation/ectasia, and 48 had CAA. Available data of 98 patients with coronary involvement were insufficient to categorize them into coronary dilation or CAA (14, 53). Also, in the remaining 21 patients, coronary involvement was described with other phrases such as "prominent" (n = 15), "increased visibility" (n = 3), "increased brightness without dilation" (n = 2), and "pericoronary hyperechogenicity" (n = 1). Matsubara et al. suggested that to maintain data analysis consistency across centers worldwide, established Z scores should be used uniformly in reporting coronary dimensions in children. Also, they suggested that even though a coronary artery may appear prominent during echocardiography, one should avoid using the term "prominent," as it is a subjective assessment, and the Z score may be within the normal range (44).
Some studies further delved into the details about the pattern of coronary artery involvement and the outcomes in the early follow-up. In the study of Theocharis et al. (52), two patients had dilations in coronary arteries on admission: (1) one in the left anterior descending (LAD) artery; and (2) the other in the right coronary artery (RCA). During the median hospital admission of 15 days (range from 11 to 25 days), the number of patients with coronary artery dilation increased to eight patients (40%); among them, two had dilations of the left main coronary artery (LMCA), four in the LAD, and two in the RCA. Further investigations with CT imaging demonstrated that 12 (60%) patients had coronary involvement in the form of coronary ectasia. Also, they stated that no distal coronary arteries were involved, and whenever coronary dilation occurred in the LMCA, its origin was involved. In another study, of six patients with coronary involvement (21%), two had coronary dilations in the RCA, and four had small CAAs: (1) two in the RCA; (2) one in the LAD; (3) and one in the LMCA. After treatment with intravenous immunoglobulin (IVIg), three had coronary Z scores < 2, and one showed improvement of RCA dilation. However, two patients still had small aneurysms with Z scores ≤ 3 (42). In a study from 55 centers in 17 European countries, 69 out of 286 patients with MIS-C had coronary involvement in any of their coronary arteries. The LMCA was the most affected artery (16.4%), and most of the coronary abnormalities persisted in the short frame of their follow-up (63).
Because there are no reliable treatment regimens for MIS-C and our knowledge about the long-term sequelae on the cardiovascular system is limited, we should consider the current guidelines about the follow-up of KD patients. They recommend repeating echocardiographic investigations at 1 - 2 weeks and again 4 - 6 weeks after treatment for uncomplicated patients. Conversely, a closer follow-up period is necessary for complicated patients with coronary Z scores ≥ 2.5 (62). Also, the long-term monitoring of patients on an occasional basis is required to determine the outcomes of MIS-C on the incidence of premature cardiovascular disease in adolescence and early adulthood.
4.3. Valvular and Pericardial Involvement
Of 33 included studies, 13 studies with 1,052 patients reported complications of heart valves. The most common one was MR, with a total frequency of 259 (24.61%) cases. Five studies categorized MR based on its severity: (1) Gaitonde et al. (38) (mild or greater: n = 6 out of 12); (2) Theocharis et al. (52) (mild: n = 10 out of 13 patients with MR; moderate to severe: n = 3 out of 13); (3) Matsubara et al. (44) (mild: n = 10 out of 13 patients with MR; moderate: n = 3 out of 13); (4) Riollano-Cruz et al. (48) (trivial to mild: n = 6 out of six patients with MR); and (5) de Farias et al. (36) (mild: n = 1 out of one patient with MR). Tricuspid regurgitation was another observed valvular involvement (n = 45, 4.28%). Although a 25% prevalence of MR among MIS-C patients is high enough to be considered a consequence of the disease itself, a prevalence of about 4% for TR is debated. Recent studies have stated that up to 60% of normal children without cardiac pathology may have mild to trivial TR (64). Moreover, compared to patients with Kawasaki disease, MIS-C patients had higher rates of MR at presentation (38). Thus, the authors suggest that MR can be considered one of the cardiac manifestations of MIS-C, while it is less likely for TR.
Nineteen studies, with a total number of 1,116 patients, mentioned pericardial involvement in their cases (n = 252, 22.58%). The outcomes of early and long-term follow-up of the patients are rarely reported. Nevertheless, the available data from longitudinal studies indicate a favorable outcome in early follow-ups. After an average follow-up of 28 days, among two patients with pericardial effusion and eight cases of MR, only two of them still had detectable MR (41). In another study, all the patients with pericardial and valvular involvement resolved after a median follow-up of 45 days (38).
4.4. Limitations
There are some limitations to this study. We included multiple case series, reducing the level of evidence. Also, there is a risk of selection bias in our review since most data came from tertiary pediatrics care centers where the patients are more complicated. One encountered problem was the lack of valid and reliable matched non-exposed cohorts in some studies. However, this shortcoming in the cohort studies did not seem to substantially alter our results or interfere with this study's aims and objectives, as we did not primarily require a specific comparison with a variable-matched group.
Furthermore, we excluded the studies lacking the desirable COVID-19 element (59, 61, 63), which could be due to the inaccuracy of data gathering in some cases. This may lead to missing the data of a considerable number of patients. Moreover, because of inter-observer variability in reporting the results of echocardiographic findings, especially for the severity of valvular involvement, we could not conduct statistical analysis, and our review was mainly descriptive. We suggest that future studies use more objective measurements for echocardiographic observations. Finally, although we used a thorough search strategy, there is a possibility of missing studies. However, we believe that the summative data are comprehensive with all these limitations in mind. We described different aspects and modalities of echocardiography in patients with MIS-C and emphasized the significance of early cardiology consultation in this disease.
There are several questions for which we could not find answers in the current literature. We could not find longitudinal studies with follow-up periods of more than two months, and in some studies, the echocardiographic findings lasted during this period. Thus, we could not determine when cardiac lesions resolve entirely. Also, the probable consequent sequelae are unknown. Another question is about the time of early follow-up echocardiography. In line with previous studies, the authors suggest that an early echocardiographic investigation is warranted after primary resuscitation. However, the data about the time of early follow-up echocardiography is not sufficient. With this in mind, the authors assume that it is reasonable to follow the current recommendations regarding Kawasaki disease in unstable patients. They stated that more frequent echocardiography (at least twice per week) should be performed for unstable patients until coronary luminal dimensions stop progressing. Also, it is recommended to repeat echocardiographic studies one to two weeks and four to six weeks after treatment (62).
5. Conclusions
In conclusion, MIS-C is a rare complication of COVID-19 in children and adolescents. The cardiovascular manifestations, ranging from cardiogenic shock, elevated cardiac biomarkers, myocardial dysfunction, valvular, pericardial, and coronary involvement, require early cardiologic consultation in the course of the disease. Echocardiographic data showed that the most common cardiac complication is LV systolic dysfunction with mildly decreased LVEF. To understand the true nature of cardiac involvement in MIS-C, there is a need to do further large-sample comprehensive echocardiographic studies, especially with 2D-STE and TDI. Also, long-term monitoring of these patients is required.