1. Background
During the widespread outbreaks of coronavirus disease (COVID-19) in late 2019, there was contradictory information about the symptoms and severity of this disease in children. Initially, the manifestation of COVID-19 was thought to be mild in many cases (1). However, the National Health Service in England warned in April 2020 that some children and adolescents with a positive test for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) developed a new range of clinical manifestations (2). Later, the Centers for Disease Control and Prevention (CDC) defined the new condition as a multisystem inflammatory syndrome in children (MIS-C) (3). Currently, the World Health Organization (WHO) and CDC have defined some criteria for diagnosing MIS-C.
The previous studies showed that, unlike severe acute COVID-19, which often occurs in children with underlying health problems, MIS-C is found in less than 1% of children with COVID-19, mainly with no previous diseases (4, 5). Although the pathophysiology of this disease is not well understood, host factors that lead to immune system dysfunction appear to play a more significant role than viral factors (5). A considerable point about MIS-C is the overlapping of some clinical features with Kawasaki disease (KD). These symptoms include fever, rash, conjunctival blood vessels dilation, and oropharynx erythema, which are common in many other infectious diseases and can make the diagnosis difficult (3, 6).
The management of each patient depends on the symptoms and course of the disease. Treatment entails fluid resuscitation, cardiac and respiratory support, empirical antibiotic coverage in admitted patients with MIS-C, and antiviral agents, such as Remdesivir in confirmed cases of COVID-19. Moreover, due to the inflammatory nature and similarity of MIS-C to KD, some benefits were observed in using immunomodulators, namely corticosteroids, tocilizumab, anakinra, and intravenous immunoglobulin (IVIG) (6).
Further studies regarding the treatment, prognostic factors, and outcomes of these patients will help clarify the various aspects of MIS-C in the future. In the present study, we investigated the clinical and laboratory findings of patients with MIS-C, which could be helpful for timely diagnosis and preventing possible complications.
2. Objectives
We aimed to evaluate the clinical and laboratory findings of MIS-C to be used for timely diagnosis and preventing possible complications.
3. Methods
This descriptive study was performed on all children and adolescents under 21 years old with MIS diagnosis who were admitted to Besat hospital as the referral center for pediatric infectious disease in Hamadan province, Iran, during September 2020-March 2021. The MIS was diagnosed based on CDC or WHO criteria for MIS diagnosis, and a total number of 47 patients were included in the study.
3.1. Case Definition
WHO defines MIS-C cases based on six criteria, including: (1) children and adolescents less than 19 years; (2) fever for 3 days or more; (3) no other plausible cause of inflammation; (4) any evidence of SARS-CoV-2 infection, such as positive antigen test, positive serology, positive SARS-CoV-2 reverse transcription-polymerase chain reaction (RT-PCR) or exposure to an individual with COVID-19; (5) multisystem involvement with at least two clinical signs, such as cardiac dysfunction, valvulitis, pericarditis, coronary abnormalities, acute gastrointestinal symptoms, evidence of coagulopathy, shock, hypotension, mucocutaneous inflammation signs, rash, or bilateral non-purulent conjunctivitis; and (6) elevated inflammatory markers, such as erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), or procalcitonin (7).
Based on CDC case definition, patients must meet all four following criteria: (1) age under 21 years; (2) no other probable diagnosis; (3) any evidence of COVID-19; and (4) all clinical features compatible with MIS-C, including fever > 38°C or a subjective fever for 24 h or longer, laboratory findings of inflammation, evidence of the involvement of at least two systems, such as cardiovascular, gastrointestinal, hematologic, respiratory, neurologic, renal, or dermatologic, and severe symptoms requiring hospital admission (8).
Age, gender, weight, past medical history, history of close contact with a known case of COVID-19, clinical manifestations, results of physical examination, laboratory findings, results of pulmonary and cardiac imaging, details of treatments, the results of COVID-19 PCR tests, and results of the serologic evaluation for COVID-19 were entered to the checklists. Clinical manifestations, physical examination, and laboratory findings were recorded twice with complete details. The first time was at admission and the second time was during hospitalization, revealing remarkably abnormal results.
Moreover, echocardiography was performed once or several times considering the condition of patients during the disease course. The first echocardiography was carried out at the presentation. If the first echocardiogram was normal, the second one was performed one or two weeks later. In case coronary artery dilation was found in the first echocardiogram second one was completed after two or three days. In case of systolic dysfunction or myocarditis, an echocardiogram was repeated according to the suggestion of a cardiologist (5). The first and the worst results of echocardiography were recorded. The results of chest X-ray (CXR) and chest computed tomography (CT) scans were also recorded. Finally, patients were evaluated in terms of meeting the CDC or WHO criteria for MIS-C diagnosis, and cases that met at least one criterion were included in the study for further analysis.
The continuous variables are presented as mean and standard deviation, while the categorical variables are shown as frequency and percentage. Fisher’s exact test, Mann-Whitney test, and paired t-test were used to compare parameters between gender and age groups (i.e., < 5, 5 - 10, and > 10 years). All statistical analyses were conducted at a significance level of 0.05. Stata software version 14.2 (StataCorp, TX, USA) was used for data analysis.
4. Results
We evaluated 47 MIS-C cases confirmed by at least one WHO or CDC criteria. All the patients fulfilled the CDC criteria, and 41 cases met both criteria. Of the total cases, 25 (53.2%) patients were male, and the median age of participants was 5.58 years with a range of 6 months to 15 years. Four individuals had a history of asthma. The PCR test was positive for SARS-CoV-2 in five cases, negative in 29 cases, and undetermined in 13 patients. Furthermore, serologic examination for SARS-CoV-2 evaluated IgM and IgG levels in 21 and 22 cases, respectively. IgM was positive in 14.3% of the assessed people, and IgG was positive in 72.7% of the reviewed cases, demonstrating the prevalence of prior SARS-CoV-2 infection in MIS-C patients.
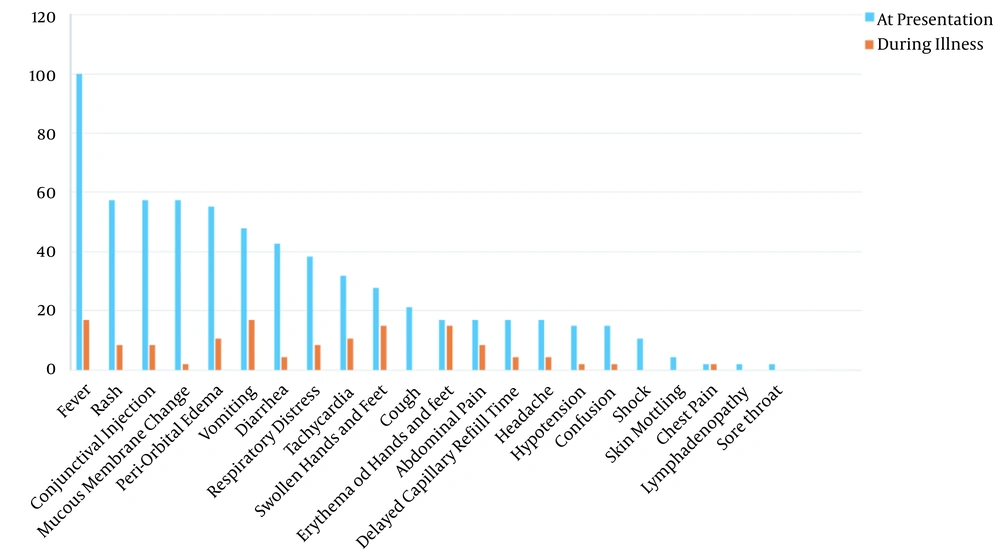
In terms of clinical manifestations, fever was detected in all cases at presentation. However, only 17% of patients continued to have a fever during hospital admission. It was found that 21.3% had a cough at presentation and all of them recovered during the following days. Just one case had chest pain during the disease course. In addition, 38.3 and 8.5% of the patients had respiratory distress at admission to hospital during illness, respectively. The hemodynamics of patients were also evaluated. At presentation, 31.9, 17, 4.3, and 14.9% had tachycardia, delayed capillary refill time, skin mottling, and hypotension, respectively. During hospitalization, these signs improved, leading to 10.6, 4.3, 0, and 2.1% representing tachycardia, delayed capillary refill time, skin mottling, and hypotension, respectively. Age and gender did not have significant relationships with the mentioned clinical manifestations. Details about other clinical symptoms are mentioned in Figure 1.
Concerning laboratory findings, CRP was elevated in 85% of cases at presentation, and it was elevated in 77% of patients during the illness. Augmented troponin was not detected in any of the patients. We observed that 40.9% of patients had lymphopenia based on their age on the first evaluation after admission, and 13.8% had lymphopenia during hospitalization. Urinalysis revealed leukocyturia in 22.6%, proteinuria in 16.1%, and hematuria in 12.9% of cases. The mean levels of other markers are mentioned in Table 1. Mean ESR (P = 0.044), prothrombin time (P = 0.043), and blood urea nitrogen (P = 0.039) were significantly different between girls and boys.
All patients were evaluated by CXR, and 66% had normal CXR. Interstitial lung abnormalities, consolidation, and pleural effusion were detected in 21.3, 6.4, and 6.4% of patients, respectively. Moreover, a chest CT scan was performed for 39 patients, and about half of them (51.3%) had normal results. Peripheral ground glass opacity, consolidation, and nodules were detected in 38.5, 23.1, and 2.6% of chest CT scans, respectively. The cardiac evaluation was performed by echocardiography for all patients after admission, and the echocardiographic examination was repeated in 38 individuals during the hospitalization period as clinically indicated. Almost half of the patients had a normal myocardial function on the first echocardiogram. Details of the first and second echocardiogram results are mentioned in Table 2. There was no significant difference between gender and age groups regarding pulmonary and cardiac imaging findings.
| Laboratory Finding | First Evaluation After Admission | The Worst Findings During Hospitalization | Normal Ranges |
|---|---|---|---|
| WBC (/µL) | 8648.94 ± 5209 | 12193.14 ± 16116.3 | 5000 - 10000 |
| Hemoglobin (g/dL) | 11.09 ± 1.6 | 11.25 ± 1.7 | 13.5 - 18 |
| Neutrophil (/µL) | 6158.96 ± 3983.5 | 6405.88 ± 3531 | Based on age |
| Lymphocyte (/µL) | 2061.00 ± 1672.8 | 2385.77 ± 1682.2 | Based on age |
| Platelets (/µL) | 222666.67 ± 139103.9 | 313566.67 ± 210188.2 | 150000 - 450000 |
| ESR (mm/h) | 36.8 ± 22.9 | 38.04 ± 29 | 1 - 15 |
| PT (s) | 15.9 ± 5.3 | 15.67 ± 3.9 | 11 - 13.5 |
| PTT (s) | 32.98 ± 12 | 31.43 ± 4.2 | 25 - 35 |
| CPK (U/L) | 204.33 ± 537.5 | 329.63 ± 600.5 | < 195 |
| LDH (U/L) | 715.68 ± 522 | 930.83 ± 766.3 | 140 - 280 |
| AST (U/L) | 46.5 ± 44.4 | 72.67 ± 113.4 | 3 - 45 |
| ALT (U/L) | 57.84 ± 82.9 | 79.39 ± 120.9 | 0 - 45 |
| Creatinine (mg/dL) | 0.72 ± 0.3 | 0.63 ± 0.2 | 0.4 - 1.3 |
| BUN (mg/dL) | 12.88 ± 10.4 | 12.84 ± 6.7 | 5 - 23 |
| Fibrinogen (g/L) | 4.23 ± 1.2 | 3.56 ± 1.4 | 2 - 4 |
| D-dimer (ng/mL) | 74.32 ± 257.4 | 3.09 ± 1.6 | < 500 |
| Albumin (g/dL) | 3.52 ± 0.6 | 3.29 ± 0.7 | 3.4 - 5.4 |
Abbreviations: WBC, white blood cells; ESR, erythrocyte sedimentation rate; PT, prothrombin time; PTT, partial thromboplastin time; CPK, creatine phosphokinase; LDH, lactate dehydrogenase; AST, aspartate aminotransferase; ALT, alanine transaminase; BUN, blood urea nitrogen.
a Values are expressed as mean ± SD.
| Echocardiogram Parameters | First Echocardiogram | Second Echocardiogram |
|---|---|---|
| Ejection fraction > 55% | 48.9 | 37.8 |
| Ejection fraction: 45 - 55% | 26.7 | 43.2 |
| Ejection fraction: 30 - 45% | 22.2 | 16.2 |
| Ejection fraction < 30% | 2.2 | 2.7 |
| Pericardial effusion | 10.6 | 7.9 |
| Coronary artery dilation | 8.5 | 7.9 |
The mean duration of hospitalization was 7.64 ± 3.2 days, and 31 patients needed to be admitted to the Intensive Care Unit (ICU) with a mean duration of ICU stay of 3.48 ± 3.5 days. Our findings showed that 59.6% of patients needed O2 support, all of which was provided by non-invasive methods, including nasal cannula and O2-mask. Antiviral treatment was administered for 27.7% of patients, and the most frequently used medication was Remdesivir. All patients received IVIG, of whom 63.8% received the medicine at the dose of 1 g/kg, and 36.2% received it at 2 g/kg. Corticosteroids were administered as low-dose (1 - 2 mg/kg) and high-dose corticosteroid pulse (10 - 30 mg/kg) in 23.4 and 70.2% of cases, respectively. It should be mentioned that 6.4% of patients did not receive any corticosteroids.
Inotropes, including dopamine and dobutamine, were administered for 38.3% of cases, and milrinone was prescribed for 19.1% of patients because they had hypotension or heart failure as a part of MIS-C. In addition, 46.8 and 29.8% of cases received aspirin and enoxaparin during hospital admission and after that. Transfusions of blood, fresh frozen plasma, and platelet were carried out for 17, 10.6, and 4.3% of patients, respectively. Finally, all patients were discharged with appropriate general conditions except one patient who expired due to severe heart failure and one patient referred to a special center for liver transplantation.
5. Discussion
As a new clinical presentation secondary to SARS-CoV-2 infection, MIS-C manifests similarly to some infectious or immunologic diseases. The diagnosis should be made based on one of the available criteria. Here, we discussed 47 cases diagnosed based on one or both of WHO and CDC criteria. Fever, elevated inflammatory markers, evidence of SARS-CoV-2 infection or exposure, and exclusion of other potential causes are similar between the two criteria.
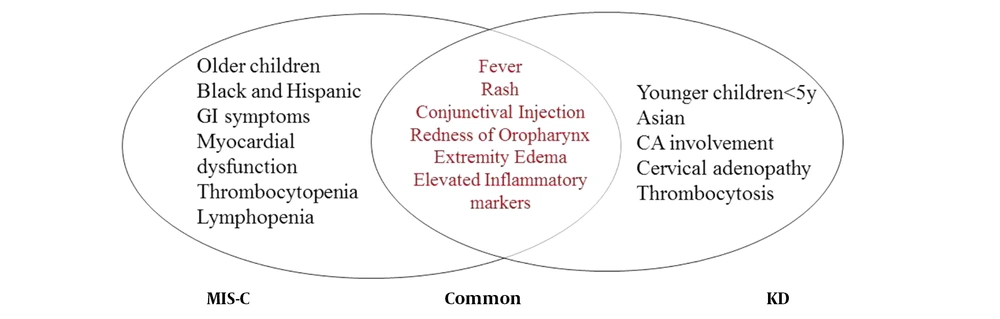
The KD and toxic shock syndrome (TSS) are two important differential diagnoses for MIS-C. It has been reported that KD is more prevalent in children under five years of age, while MIS-C frequently affects older children and adolescents (2, 3). Furthermore, gastrointestinal symptoms, cardiovascular abnormalities, and shock are more common in MIS-C than classic KD (9). Shock is an unusual presentation for KD except for the rare cases of Kawasaki shock syndrome. Unlike KD, in MIS-C, inflammatory markers tend to elevate more, and there is a more significant reduction in absolute lymphocyte and platelet counts (5). In addition, KD has been more common in East Asian children, whereas MIS-C appears to affect Hispanic, African American, and Afro Caribbean descent (9). Ultimately, a history of exposure to and evidence of COVID-19 are important clues to differentiate these diseases (5). Therefore, if the patient fulfills the criteria for KD diagnosis and has evidence of SARS-CoV-2 infection or exposure, we should consider the patient as MIS-C overlapping with KD (5). We summarized similarities and differences between MIS-C and KD, as the main differential diagnosis, in Figure 2.
The TSS is another important differential diagnosis. Fever, rash, and hypotension must be present for TSS diagnosis (10). Fever is also mandatory for MIS-C diagnosis, while rash and hypotension may not be present. However, if the patient suspected of MIS-C fulfills the criteria for TSS, antibiotic treatment should be started to cover possible staphylococcal infection.
In the current study, 53.2% of patients were male, and the median age of participants was 5.58 years with a range of 6 months to 15 years. In a similar study by Kaushik et al., 61% of patients were male, and the median age of participants was 10 years (11). Moreover, another similar study in Iran by Mamishi et al. reported that the median age of patients was 7 years with a range of 10 months to 17 years (12). The median age in both studies was higher than in our study. However, approximately half of the patients in all studies were male. There was no remarkable underlying disease in the MIS-C cases of our study except for a history of asthma in four cases. However, studies on children with severe COVID-19 showed that many cases had an underlying disease (13, 14).
Common clinical manifestations in our study were fever (100%), rash (57.4%), conjunctival injection (57.4%), mucous membrane changes (57.4%), periorbital edema (55.3%), vomit (47.8%), and diarrhea (42.6%). Another study in Iran reported fever (91%), abdominal pain (58%), mucocutaneous rash (53%), nausea/vomit (51%), conjunctivitis (51%), as well as hands and feet edema (40%) as the most common clinical manifestations in MIS-C cases (12). Fever was the most common presentation in both studies, while gastrointestinal involvement was more common in the study by Mamishi et al. (12). An investigation in the United States of America declared that 92% of MIS-C cases had gastrointestinal involvement, which was higher than our results (3).
In the present study, 55.3% of patients had periorbital edema, and 27.7% also had swollen hands and feet. According to another study in Iran, edema was a common presentation (12). We expect patients with fever and gastrointestinal involvement (diarrhea and vomiting) to be dehydrated, and edema is not expected. We can conclude that simultaneous fever, diarrhea, vomit, and limb or periorbital edema in children is a key feature of MIS-C diagnosis when we are in COVID-19 pandemic, and it should be considered in addition to the other causes of renal involvement.
In terms of laboratory findings, 42.6, 85, and 40.9% of patients in our study had elevated ESR, increased CRP, and lymphopenia based on their age, respectively. Another study on MIS-C patients in Iran by Mamishi et al. reported augmented ESR, raised CRP, and lymphopenia in 35.5, 67, and 54% of patients, respectively (12). The rate of elevated ESR or CRP was higher in our study, while lymphopenia was more prevalent in the mentioned research (12). The rise in inflammatory markers was also reported in several studies on MIS-C patients (13-15).
In our study, urinalysis detected leukocyturia in 22.6%, proteinuria in 16.1%, and hematuria in 12.9% of cases, while Mohkam et al. reported proteinuria in 46% and hematuria in 23% of patients admitted due to COVID-19 (16). Comparison of the results indicates that renal involvement was observed more frequently in children with COVID-19 than MIS-C.
All patients were evaluated by CXR, and results were interpreted according to the Radiological Society of North America (RSNA) (17). It was observed that 66% of CXR results were normal, and interstitial lung abnormality, consolidation, and pleural effusion were detected in 21.3, 6.4, and 6.4% of patients, respectively. Chest CT-scan was performed for 39 patients showing that 51.3% were normal. However, peripheral ground glass opacity, consolidation, and nodules were detected in 38.5, 23.1, and 2.6% of chest CT scans, respectively. CT scans were also interpreted according to the RSNA guideline. Soltani et al., in their study on patients admitted due to COVID-19, detected ground glass opacity, consolidation, and nodules in 73.1, 42.3, and 15.4% of participants, respectively (15). The difference between COVID-19 and MIS-C patients in these two studies, both conducted in the west of Iran, demonstrates that pulmonary involvement is less common in MIS-C patients than patients with typical SARS-CoV-2 infection. According to the RSNA guideline, pleural effusion is an atypical manifestation of COVID-19 pulmonary involvement. However, it was detected in 6.4% of our patients, showing that pleural effusion is more common in MIS-C than in COVID-19.
We evaluated all participants by echocardiography, finding that 48.9% had a normal cardiac function, 26.7% had mild dysfunction, 22.2% had moderate dysfunction, and 2.2% had severe dysfunction. Pericardial effusion and coronary artery dilation were observed in 10.6 and 8.5% of patients, respectively. In the study by Mamishi et al., 56% had cardiac involvement, of whom 31% had coronary artery dilation (12). Another study reported that 17% of MIS-C patients had coronary artery dilation (18). The rate of coronary artery dilation as a complication of MIS-C was lower in our study in comparison to other similar ones. This difference can be due to the timely diagnosis and treatment of the syndrome in our patients, leading to lower complications.
The individuals in the present research were treated according to Iranian guidelines (1). All the patients in the current study received IVIG, 63.8% received medicine at the dose of 1 g/kg, and 36.2% received the medication at 2 g/kg. All our patients had a proper response to treatment with no further cardiac complication on follow-up, except for one who was expired. In the study by Mamishi et al., 47% of patients received IVIG at the dose of 2 g/kg (12). According to our results, it could be concluded that the dose of 1 gr/kg is enough for treatment, and there is no need for the dose of 2 g/kg considering its adverse effects as a plasma-derived product. In line with other similar studies, our study showed a low mortality rate in MIS-C patients. It is noteworthy that the mortality rate was 5% in one of the Iranian studies (12, 13, 19, 20).
5.1. Conclusion
Gastroenteritis is usually associated with dehydration. As a result, edema is an unusual finding, and we can conclude that in the case of gastroenteritis along with limb or periorbital edema in children, there are two important differential diagnoses, namely hemolytic uremic syndrome and MIS-C. The MIS-C diagnosis should be highly suspicious regarding the current COVID-19 pandemic and high prevalence of gastrointestinal symptoms in MIS-C induced by COVID-19. Furthermore, it could be concluded that a child with gastroenteritis and rash, hypotension, or shock suspected to TSS should be examined for SARS-CoV-2 infection along with essential measures for TSS treatment because MIS-C and TSS may represent similar manifestations. In addition, patients with signs or symptoms of typical or atypical KD should be screened for SARS-CoV-2 infection due to highly similar presentations of these two diseases.