1. Background
Ulcerative colitis (UC) is a chronic non-specific inflammatory disease of the colon. Most of the lesions arise in the rectum and are continuous. In severe cases, the entire colon can be involved. In recent years, the incidence of UC in children has increased worldwide. The incidence of UC in Europe has increased from 1.6/10000 to 4.1/100000 (1), and the incidence of UC in China has increased from 0.2/1000000 to 2.8/1000000 (2).
Steroids are the first choice for mild, moderate, and severe active UC that does not respond to 5-aminosalicylic acid (5-ASA) for induction treatment. Steroids can promptly relieve symptoms, such as hematochezia, diarrhea, and abdominal pain (3). It has been shown clinically that 16% - 34% of patients have steroid resistance and approximately 22% of patients have steroid dependence (4). In recent years, the therapeutic effect of biological agents on UC has become a focus of research. Among the biological agents, infliximab (IFX), a human-mouse chimera anti-tumor necrosis factor-alpha (TNF-α) monoclonal antibody, is the earliest and most widely used biological agent worldwide. Active Ulcerative Colitis Trials 1 and 2 (ACT1 and ACT2), such as RUTGEERTS (5), showed that IFX has a significant effect on patients with moderate-to-severe UC, including patients with steroid dependence or resistance, and some patients can be freed from steroid dependence. There are still many questions about IFX in the treatment of UC in children, such as the treatment duration and the timing of withdrawal, which need to be further studied.
2. Objectives
The current study summarizes the clinical efficacy of nine children with steroid-refractory UC treated with IFX in our hospital and the clinical effects of IFX treatment in children with moderate-to-severe UC for improved treatment of refractory UC.
3. Methods
3.1. Participants of the Study
The clinical data from nine children with steroid-refractory (steroid-resistant or -dependent) moderate-to-severe UC who were treated with IFX in our hospital from January 2015 to the present were collected. The diagnosis of UC conformed to the diagnostic criteria of UC in children per the “Consensus on diagnostic guidelines for inflammatory bowel disease in children”, formulated by the Children’s Inflammatory Bowel Disease Collaborative Group of the Pediatric Association of the Chinese Medical Association (6). The definition of steroid-refractory UC was as follows: Steroid lack of effectiveness indicates no response to treatment with prednisone or an equivalent to 0.75 mg/(kg·d) for > 4 weeks or to intravenous corticosteroid treatment within 5 - 7 days; steroid dependence refers to a patient with UC that can be maintained in remission, the prednisone dose cannot be reduced to 10 mg/d after 3 months of treatment, or UC recurs within 3 months after discontinuing the steroid (naturally or after drug treatment and UC is in remission, hematochezia and diarrhea reoccur) (7).
This is retrospective study, we collected clinical data from the children, including gender, age, disease course, clinical manifestations, scope of lesions, clinical type, disease activity, and presence or absence of extraintestinal manifestations, laboratory examinations, endoscopic examinations, and histologic results. The range of lesions was determined using the Paris classification (8). Disease activity was based on the Pediatric Ulcerative Colitis Activity Index (PUCAI), as follows (9): PUCAI < 10 is the remission period; PUCAI = 10 - 34 is a mildly active period; PUCAI = 35 - 64 is a moderately active period; and PUCAI ≥ 65 is a severely active period. The Ulcerative Colitis Endoscopic Index of Severity (UCEIS) was used for endoscopic scoring as follows (10): 0, mucosal healing; 1 - 3, mild active period; 4 - 6, moderate active period; and 7 - 8, severe active period. The laboratory tests include white blood cell count (WBC), hemoglobin (Hb), platelet count (PLT), C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR)
3.2. Dosage and Route of Administration of IFX
IFX was administered by an intravenous infusion at a dose of 5 mg/kg. IFX was used as induction therapy at 0, 2, and 6 weeks, and consolidation treatment was administered every 8 weeks thereafter.
3.3. Effectiveness Evaluation Indicators
Patients were evaluated for clinical symptoms, and disease activity before and after IFX treatment at the end of the induction period (week 14), at week 30, and at week 54. Colonoscopy were performed at week 14, week 30, and week 54 (11). Clinical remission refers to the resolution of clinical symptoms, including < 3 stools per day, no blood in the stool, no abdominal pain, and a PUCAI < 10. A clinically significant response is defined by a PUCAI decrease of at least 20 points or entering remission (9). Mucosal healing refers to repair of inflamed sites visualized during endoscopy. Healthy colorectal mucosa is not friable, has no active bleeding sites, no erosions, and no ulcers (12). According to the International Organization for the Study of Inflammatory Diseases (IOIBD), mucosal healing refers to the Mayo score or a UCEIS of 0. A score ≤ 1 is considered endoscopic remission and an endoscopic response is defined as a reduction of at least 1 point in the Mayo score or a reduction of at least 2 points in the UCEIS (11). We also noted the loss of response (LoR) and adverse drug reactions during IFX treatment. The LoR to IFX is divided into three categories: (1) Primary non-response (PNR); (2) secondary non-response (SNR); and (3) secondary-primary non-response or failure of re-induction (13).
3.4. Usage of Immunosuppressants
Nine with steroid-refractory UC were treated with azathioprine 2 - 2.5 mg/kg·d before IFX treatment. It continued after initiation.
3.5. Statistical Analysis
Statistical analyses were performed with SPSS 22.0 (IBM Corp., Armonk, NY, USA). Non-normally distributed data are expressed as [M (Q1, Q3)]. The rank-sum test of variance was used to compare differences of data before and after treatment. The Mann-Whitney U test was used to compare differences between patients with a significant response and those with a non-response. P-values < 0.05 were considered statistically significant.
3.6. Ethical Statement
The study protocol was approved by the Ethics Committee of Shengjing Hospital of China Medical University (2021PS517K).
4. Results
4.1. Basic Clinical Features
There were nine children in this study, including six boys and three girls, with an average age of 8 years (age range, 3 - 12 years). The average course of disease was 2 months (range, 0.5 - 24 months). The clinical manifestations of UC were as follows: Bloody stool, 7 (77.8%); diarrhea, 7 (77.8%); abdominal pain, 7 (77.8%); and fever, 1 (11.1%). The clinical types of UC were as follows: Initial onset type, 7 (77.8%); and chronic relapse type, 2 (22.2%). The extent of lesions was as follows: Total colon, 7 (77.8%); and extensive lesions, 2 (22.2%). Five children (55.6%) had severe active disease and 4 children (44.4%) had moderately active disease. Two children had extraintestinal manifestations of UC, including one child with juvenile idiopathic arthritis and one child with recurrent oral ulcers (Table 1).
| Variables | Values |
|---|---|
| Age (y) | 8 (3 - 12) |
| Gender | |
| Male | 6 (66.7) |
| Female | 3 (33.3) |
| Duration (months) | 2 (0.5 - 24) |
| Clinical symptoms | |
| Bloody stool | 7 (77.8) |
| Diarrhea | 7 (77.8) |
| Abdominal pain | 7 (77.8) |
| Fever | 1 (11.1) |
| Clinical type | |
| Initial onset | 7 (77.8) |
| Chronic relapse | 2 (22.2) |
| The extent of lesions | |
| Pancolitis | 7 (77.8) |
| Extensive lesions | 2 (22.2) |
| Disease activity | |
| Severe | 5 (55.6) |
| Moderate | 4 (44.4) |
| Extraintestinal manifestations | 2 (22.2) |
a Values are expressed as No (%).
4.2. Laboratory Testing
Of the nine children with UC, the WBC count was increased in five (55.6%), the Hb concentration was decreased in eight (88.9%), the PLT count was increased in seven (77.8%), the CRP level was increased in four (44.4%), and four (44.4%) had an elevated ESR. The albumin level was decreased in 4 children (44.4%).
4.3. Endoscopy and Histology
Based on the UCEIS system, 3 children (33.3%) had mild activity, 5 children (55.6%) had moderate activity, and 1 child (11.1%) had severe activity. The pathologic manifestations included an inflammatory cell infiltration in 9 children (100.0%), cryptitis in 2 children (22.2%), and crypt abscesses in 2 children (22.2%).
4.4. Evaluation of IFX Treatment Effects
The median number of IFX administrations was 6 (Q1 6, Q3 10) and the median time from diagnosis of UC-to-the administration of IFX was 4 months (Q1 1, Q3 7).
At week 14, a clinically significant response was achieved in 6 children (66.7%, 5 of whom achieved clinical remission and the PUCAI of the other child dropped by 30 points). Four of the 6 children had mucosal healing (66.7%) and 2 had an endoscopic remission (33.3%).
At week 30, one child had not reached this point of treatment as of this writing. Among the remaining 5 children with a clinically significant response, 3 (60%) had a sustained clinical remission and mucosal healing after 6 courses of IFX, then discontinued IFX. One child (20%) with an endoscopic remission had mucosal healing, 1 (20%) had mild disease.
At week 54, among the 6 children with a clinically significant response at week 14, five were in clinical remission with mucosal healing, and 1 has not reached week 54 of treatment (Table 2).
| Variables | Values a |
|---|---|
| Median number of IFX administrations (M (Q1, Q3)) | 6 (6,10) |
| Median time from diagnosis of UC-to-the administration of IFX (months, M (Q1, Q3)) | 4 (1,7) |
| At week 14 | |
| Clinically significant response | 6 (66.7) |
| PUCAI < 10 | 5 (83.3) |
| PUCAI decrease of at least 20 points | 1 (16.7) |
| UCEIS = 0 | 4 (66.7) |
| UCEIS ≤ 1 | 2 (33.3) |
| Loss of response | 3 (33.3) |
| At week 30 | |
| Clinically significant response | 5 (55.5) |
| PUCAI < 10 | 4 (80) |
| PUCAI = 10 - 34 | 1 (20) |
| UCEIS = 0 | 4 (80) |
| UCEIS ≤ 1 | 1 (20) |
| At week 54 | |
| Clinically significant response | 5 (55.5) |
| PUCAI < 10 | 5 (100) |
| UCEIS = 0 | 5 (100) |
Abbreviations: PUCAI, Pediatric Ulcerative Colitis Activity Index; UCEIS, The Ulcerative Colitis Endoscopic Index of Severity.
a Values are expressed as No (%) unless otherwise indicated.
Of the 3 children who did not have a clinical response at 14 weeks, 1 child had a clinical remission after changing treatment regimens. The remission was not achieved in the other two children at the end of the observation period.
4.5. Evaluation of Laboratory Indicators
The Hb concentration in six children who were in a clinically significant response was increased compared with the pre-treatment Hb concentration, while the PLT count and CRP level were decreased pre-treatment (all P < 0.05). The WBC count and the ESR were decreased compared with pre-treatment, but the difference was not statistically significant (P > 0.05; Table 3).
| Case Number | WBC (× 109/L) | Hb (g/L) | PLT (× 1012/L) | CRP (mg/dL) | ESR (mm/h) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | Before | After | |
| 1 | 12.40 | 4.70 | 108 | 125 | 385 | 344 | 10.90 | 1.00 | 48.00 | 7.00 |
| 2 | 10.50 | 7.10 | 121 | 122 | 396 | 285 | 8.14 | 1.00 | 8.00 | 4.00 |
| 3 | 23.33 | 17.54 | 94 | 115 | 417 | 397 | 111 | 2.12 | 24.00 | 8.00 |
| 4 | 17.54 | 13.33 | 109 | 116 | 340 | 326 | 6.06 | 1.36 | 50.00 | 8.00 |
| 5 | 6.30 | 10.60 | 94 | 108 | 491 | 432 | 6.06 | 2.02 | 10.00 | 2.00 |
| 6 | 15.50 | 13.63 | 88 | 94 | 391 | 393 | 21.70 | 2.00 | 2.00 | 29.00 |
| Z | -1.363 a | -2.201 a | -1.992 b | -2.201 b | -1.363 b | |||||
| P | 0.173 | 0.028 c | 0.046 c | 0.028 c | 0.173 | |||||
a Base on rank –
b Base on rank +
c P < 0.05 vs. before treatment.
During the administration of IFX, 5 children (55.6%) had a LoR to IFX, 3 (60%) had a PNR, and 2 (40%) had a SNR. Among the 3 children with a PNR, IFX was discontinued in one and replaced with another biological agent (adalimumab) in combination with an immunosuppressive agent (azathioprine) to achieve clinical remission at week 54. Because colonoscopy was declined, healing of the mucosa was not determined (n.b., no colonoscopy was performed at the time, but a colonoscopy was performed 2 years later and had mucosal healing). The IFX maintenance treatment interval was shortened to 6 weeks in one child. After re-administration of intravenous glucocorticoids combined with azathioprine, the PUCAI of this child dropped to 10 points at week 30. At week 54, the PUCAI increased again to 35 points and the UCEIS was 4 points. One child received enteral nutrition and intravenous cyclosporine (4 mg/kg·d) and the IFX treatment interval was shortened to 4 weeks. The child had poor compliance and irregular IFX treatment. At week 30, the child had a PUCAI score of 45 and a UCEIS score of 3. Both children with an SNR were tested for trough levels of IFX and anti-antibody activity, and other optimizing treatments were given (i.e., the IFX treatment interval was shortened and the amount of IFX was increased), combined with azathioprine orally to achieve clinical relief and mucosal healing.
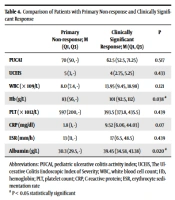
A comparison of the difference between patients with a primary non-response and a clinically significant response showed that the Hb concentration and albumin level were significantly different (Table 4).
| Primary Non-response; M (Q1, Q3) | Clinically Significant Response; M (Q1, Q3) | P | |
|---|---|---|---|
| PUCAI | 70 (50, -) | 62.5 (52.5, 71.25) | 0.517 |
| UCEIS | 5 (3, -) | 4 (2.75, 5.25) | 0.433 |
| WBC (× 109/L) | 8.0 (7.4, -) | 13.95 (9.45, 18.98) | 0.121 |
| Hb (g/L) | 83 (56, -) | 101 (92.5, 112) | 0.038 a |
| PLT (× 1012/L) | 597 (200, -) | 393.5 (373.8, 435.5) | 0.439 |
| CRP (mg/dl) | 1.8 (1, -) | 9.52 (6.06, 44.03) | 0.07 |
| ESR (mm/h) | 13 (11, -) | 17 (6.5, 48.5) | 0.439 |
| Albumin (g/L) | 30.3 (29.5, -) | 39.45 (34.58, 43.38) | 0.020 a |
Abbreviations: PUCAI, Pediatric Ulcerative Colitis Activity Index; UCEIS, The Ulcerative Colitis Endoscopic Index of Severity; WBC, white blood cell count; Hb, hemoglobin; PLT, platelet count; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate
a P < 0.05 statistically significant
One child developed urticaria during the third intravenous infusion of IFX. After intravenous dexamethasone, the IFX infusion was slowed and the urticaria resolved.
5. Discussion
The treatment goals for children with UC are to induce and maintain clinical remission and mucosal healing, promote growth and development, improve the quality of life, and control adverse drug reactions to a minimum level (6). The main treatment drugs for children with UC include 5-ASA, steroids, and immunosuppressants. The oral preparations of 5-ASA include salazosulfapyridine and mesalazine; the rectal preparations of 5-ASA include enemas and suppositories. Immunosuppressants mainly include thiopurines and methotrexate. IFX is most often used as a rescue treatment and is considered when there is at least one non-response or intolerance to conventional treatment drugs (14).
TNF-α is a pro-inflammatory cytokine with a wide range of effects and plays an important role in the UC inflammatory cascade. Anti-TNF-α biological agents inhibit the binding of TNF-α with the receptor to block the development of inflammation and achieve anti-inflammatory effects (15). IFX is a TNF monoclonal IgG antibody that contains approximately 75% human protein and 25% mouse protein. IFX was approved by the Food and Drug Administration (FDA) for the clinical treatment of UC in 2006 (16). Studies have shown that UC patients have clinical improvement during the second week of IFX treatment, and the clinical response, clinical remission, and mucosal healing rates at 8, 30, and 54 weeks were higher than the placebo group (5). Fratila and Craciun (17) performed endoscopic mucosal healing and histologic observations on seven patients with moderate-to-severe refractory UC before and after the first IFX treatment and reported that intestinal epithelial organelles were significantly improved in morphology and function, mucus secretion was normal, and chorionic tissue had been restored.
The current study showed that the therapeutic effect of IFX in children with UC was positive. Among 9 children with steroid-refractory moderate-to-severe UC, 6 (66.7%) achieved a clinically significant response, 4 (66.7%) children had mucosal healing, and 2 (33.3%) reached an endoscopic remission at week 14. After the administration of IFX, the Hb concentration of children in clinical remission was increased compared to pre-treatment, and PLT count and CRP level were decreased, all of which were statistically significant differences. These results suggest that IFX can be used as a salvage treatment for refractory UC. The decreased WBC count and ESR were not statistically significant, which is thought to be related to the small sample size in the current study.
Problems remain regarding IFX treatment of UC in children. In this study, nine children with UC were treated with IFX, and five children (55.6%) had a LoR, three (60%) had a PNR, and two (40%) had a SNR. Because IFX is still the most widely used biological agent in the treatment of Crohn’s disease and UC, in order to obtain the optimal therapeutic effect, it is important to study the LoR to IFX. LoR is divided into a PNR and SNR, and failure to respond after drug withdrawal. The most common definition of a PNR is that the clinical symptoms and signs have not improved after IFX induction therapy, including complete failure to respond to IFX and a partial response without complete remission (18). There are different opinions about the time to determine primary unresponsiveness. Some scholars believe that the judgment of PNR depends on the severity of IBD. Patients with severe disease need to be assessed earlier and were evaluated after the second infusion of infliximab. Patients with mild disease or a partial response to the drug should be evaluated after completion of induction therapy (13, 19, 20). A SNR refers to the deterioration and recurrence of UC in children who respond in the initial stage of biological treatment (18). At present, there is limited research on the specific mechanism underlying the occurrence of an IFX LoR. Various factors, such as drug concentration, drug clearance rate, and a non-TNF-driven inflammatory process, may all be related to the LoR (20). The trough concentration of the drug refers to the lowest point of the drug concentration before the next administration of the drug, which is also known as the lowest effective drug concentration. A low serum trough concentration of IFX is closely related to IFX treatment failure. Studies have shown that the most suitable target serum trough concentration for IFX in the treatment of IBD is 3 - 7 μg/mL (21); research involving trough concentrations has mostly involved adults. Whether there are different optimal trough concentrations for IFX in pediatric patients and whether the effective trough concentrations of IFX in Crohn’s disease and UC are consistent await further study. At present, it is believed that rapid clearance of infliximab in UC, especially acute severe colitis (ASC), intensification of induction regimen is often needed. Doses of infliximab up to 10 mg/kg/dose may be considered and may be given more frequently than usual (22). In our study, the dosage of IFX in the induction stage was the standard amount. This is an important reason for the failure of IFX in our study, and it also indicates that the drug level during induction can determine the efficacy of maintenance. IFX clearance is influenced by a variety of factors, including body mass index, gender, presence or absence of combination immunosuppressant use, serum albumin concentration, intestinal inflammatory burden, and disease behavior (e.g., chronically active UC) (23, 24). In our study, we compared the clinical and endoscopic scores and laboratory indicators of children in the primary non-response and clinically significant response groups. The decrease in hemoglobin concentration and albumin level were significantly different between the two groups. In the future we hope to obtain more samples to study the factors influencing primary unresponsiveness in children with UC and whether there are differences between children and adults.
Due to medical condition limitations, the serum trough concentration and anti-antibody of IFX were carried out late in our center. The three primary unresponsive children in this study could not be monitored for drug treatment during the observation period. Two children with a SNR were optimized for the treatment plan based on the IFX trough concentration and anti-antibody level, and finally achieved clinical remission and mucosal healing after adjusting the dose of IFX or shortening the interval between use. Therefore, the optimized treatment plan may have therapeutic guidance value for UC children with a SNR.
The timing and duration of IFX administration in children with UC needs further study. At present, IFX is listed in the guidelines of the American College of Gastroenterology, Practice Parameters Committee as a class A drug recommended for the treatment of moderate-to-severe steroid-resistant or -dependent UC (25). Based on the expert consensus of children with inflammatory bowel disease, IFX is also used as a rescue treatment for UC (6). In the current study, the median time at which IFX was administered to the nine children with moderate-to-severe steroid-refractory UC was 4 months after diagnosis, and five children failed to respond, including three children with a PNR. The times to initial application of IFX in these three children were 12 months (n = 2) and 7 months after diagnosis, which is significantly longer than other children with better effects. Whether the occurrence of these failures was related to the time from diagnosis to the administration of IFX requires further studies with a larger sample size. In addition, follow-up time of this study was up to the week 54 of IFX. In fact, during the subsequent follow-up, among the 6 children who were in clinical remission at week 54, five had sustained clinical remission until now, but 2 had endoscopic mucosal edema. One child had a clinical and endoscopic relapse, whether IFX was used regularly for a long time or stopped after a specified period of time. It seems that the time course for IFX treatment and regular medication is not necessarily related to UC improvement. Therefore, the specific course of treatment and the timing of discontinuation of IFX in the treatment of children with UC warrant further clinical and basic investigations.
In summary, this study showed that IFX is effective in the salvage treatment of children with moderate-to-severe steroid-refractory UC, and patients can achieve partial clinical remission and mucosal healing. Children with clinical remission have decreased inflammatory indicators and better Hb recovery. In the process of treatment, however, there are still issues, such as the timing of use, the specific course of treatment, and how to avoid the occurrence of a LoR. Because this study only involved a small number of cases, a large-scale multi-center clinical research is essential.