1. Background
Acute respiratory distress syndrome (ARDS) is an acute inflammatory lung injury caused by a variety of etiologies, clinically characterized by progressive hypoxemia and respiratory distress (1), which conventional ventilator support is ineffective. ARDS is one of the common causes of death in children hospitalized in the pediatric intensive care unit (PICU). The overall case fatality rate is as high as 25% (2), and the case fatality rate is higher in severe cases. The 2015 consensus pointed out that for severe ARDS children (with impaired gas exchange despite conventional lung protection measures), extracorporeal membrane oxygenation (ECMO) can be considered (3). ECMO draws the blood out of the body, effectively absorbs oxygen and excretes CO2 through the membrane lung, and then returns the oxygenated blood to the body through an artificial pump to provide long-term cardiopulmonary support to the patient (which is conducive to the improvement of lung function and cardiac function) and gain valuable time for the recovery of lung and heart function. It has become the preferred salvage treatment for children with severe ARDS when lung-protective ventilation strategies fail (4). This article reported the experience of using ECMO in critically ill children with ARDS of different ages and diagnoses to provide a reference for clinical practice.
2. Methods
2.1. Study Design and Participants
Children with ARDS admitted to the PICU of a tertiary children’s hospital in China between January 2017 and March 2020 were enrolled in this study. The clinical manifestations of all children were consistent with the diagnostic criteria for ARDS (5): protective ventilator-assisted ventilation not being able to maintain basic oxygenation in children with respiratory failure, with the following performance parameters: 1 severe hypoxemia [arterial partial pressure of oxygen (PaO2)/FiO2 < 80 ~100 mm Hg, 1 mm Hg = 0.133 kPa], high positive end-expiratory positive pressure (PEEP) ventilation (usually > 15 cm H2O, 1 cm H2O, > 0.098 kPa) for potentially reversible respiratory failure, 2 severe hypoxemia, and/or high inspiratory plateau pressure (> 35 - 45 cm H2O).
All patients received venous-arterial ECMO (veno-arterial, V-A ECMO) mode and were treated with heparin-coated ECMO-specific intravenous 8 - 19 Fr. catheter. Heparin, 1 mg/kg, was intravenously injected 2 minutes before intubation. Heparin was pumped at 5 - 15 u/kg/h after ECMO establishment. According to the results of monitoring the activated clotting time (ACT) and coagulation function, the heparin maintenance amount was adjusted to maintain the ACT at 180 - 220 seconds. The ventilator uses a protective strategy during ECMO support to reduce and prevent complications. The respiratory support used a synchronous intermittent command ventilation mode with parameters adjusted between 40% and 50% for FiO2 and 20 to 25 beats per minute for the respiratory rate, tidal volume (4 - 5 mL/kg), PEEP (5 - 9 cm H2O), and peak inspiratory pressure (PIP; 10 - 15 cm H2O, on the basis of PEEP). The auxiliary flow rate was adjusted according to the hemodynamics and oxygenation index (OI) of the child, and the range is 50 - 120 mL/min/kg. Between 40% and 60%, the arterial oxygen saturation was not less than 95%.
The respiratory status of children during ECMO operation was assessed daily by chest X-rays, blood gas analysis results, and hemodynamic parameters. During ECMO operation, all children were placed in a prone position; the ventilation was provided twice a day for 3 hours. Fiberoptic bronchoscopy and lavage were performed according to the lung condition of the children; 2 children with bronchitis were treated once every other day. The auxiliary flow was gradually reduced according to the recovery of respiratory function and chest X-rays. When the auxiliary flow was reduced to 10% - 20% of the total flow, we tried to evacuate ECMO. First, the parameters of the ventilator were adjusted to ECMO after evacuation. After adjusting the required parameters, the gas flow of the oxygenator was clamped, and the observation took about 30 - 60 minutes to ensure that the child’s respiratory function was stable. Generally, the experiment was started 2 days before the planned evacuation. The oxygenation was maintained in the normal range during each attempt. The ECMO cannula was removed, and the neck vessels were ligated.
All children were treated with antibiotics, anti-infection, strict control of fluid volume, low-dose hormones and c-cells, and high-condition ventilator-assisted therapy. Five children received high-frequency vibration ventilation and partial pressure oxygen (which was still insufficient to maintain oxygen saturation).
2.2. Ethical Consideration
This study was approved by the Institutional Review Board of the Children’s Hospital, Zhejiang University School of Medicine (code: 2021-IRB-050).
2.3. Data Collection
After receiving consent from the children’s parents/guardians, the ECMO treatment was performed with the joint efforts of the extracorporeal circulation department, cardiac surgery, and ICU. Data collection was completed in February 2020. Data were collected from medical records. General characteristics included gender, age, body weight, ventilator use before ECMO establishment, ECMO operation mode, ECMO operation hours, number of fiberoptic bronchoscopy assisted treatments, days on the ventilator after ECMO evacuation, total days on the ventilator, days of hospitalization, pathogen infection, primary disease/underlying disease, complications during ECMO therapy, comparison of monitored values of children 1 hour before and after ECMO establishment, analysis of the causes of complications during ECMO treatment in ARDS children.
2.4. Data Analysis
Collected data were analyzed using SPSS version 23 (SPSS Inc., Chicago, Ill, USA). Data are expressed as percentages, mean, or median. The paired-sample t-test was used to compare the monitoring values of children 1 hour before and after the establishment of ECMO. P values less than 0.05 were considered statistically significant.
3. Results
3.1. General Information
Study participants (8 males and 8 females) who received ECMO adjuvant treatment ranged in age from 3 days to 10 years (average of 3.25 ± 2.87 years old) with body weight ranging from 12 to 19.5 kg (average of 14.13 ± 7.71 kg). The ECMO assist time was 92 - 759 hours (average of 255.13 ± 172.38 hours). The ventilator was used before ECMO application. One patient was on a ventilator for 14 days before receiving an ECMO application; all other children were on ventilators for 7 days before receiving ECMO. The average time of treatment with a ventilator before ECMO was 62.28 ± 90.36 hours. Adenovirus infection was the most common infection, followed by influenza A infection. One of the children tested negative for all pathogens, but the virus infection was highly suspected according to clinical manifestations. The specific pathogen infections are shown in Table 1.
| Comorbidity | Complication Rate (Example, %) |
|---|---|
| Adenovirus | 7 (43.7) |
| Influenza A virus | 5 (31.2) |
| Respiratory syncytial virus | 2 (12.5) |
| Mycoplasma pneumoniae | 2 (12.5) |
| Cytomegalovirus infection | 1 (6.2) |
| Pneumocystis carinii | 1 (6.2) |
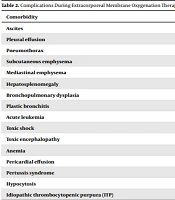
A total of 16 patients with ARDS received ECMO supportive care between January 2017 and March 2020. All patients had comorbidities before receiving ECMO; 6 patients presented with pleural effusion, pneumothorax, mediastinal emphysema, and 14 patients without the primary disease, including 1 with acute leukemia and 1 with idiopathic thrombocytopenic purpura (ITP) underlying disease. The specific patients’ complications are shown in Table 2.
| Comorbidity | Complication Rate (Example, %) |
|---|---|
| Ascites | 5 (31.2) |
| Pleural effusion | 15 (93.7) |
| Pneumothorax | 7 (43.7) |
| Subcutaneous emphysema | 5 (31.2) |
| Mediastinal emphysema | 7 (43.7) |
| Hepatosplenomegaly | 2 (12.5) |
| Bronchopulmonary dysplasia | 1 (6.2) |
| Plastic bronchitis | 2 (12.5) |
| Acute leukemia | 1 (6.2) |
| Toxic shock | 1 (6.2) |
| Toxic encephalopathy | 2 (12.5) |
| Anemia | 1 (6.2) |
| Pericardial effusion | 1 (6.2) |
| Pertussis syndrome | 2 (12.5) |
| Hypocytosis | 1 (6.2) |
| Idiopathic thrombocytopenic purpura (ITP) | 1 (6.2) |
Sixteen children received high-condition ventilator parameters before the ECMO application. The arterial oxygen partial pressure and oxygen saturation were improved in all children. Five children had a heartbeat within 2.50 to 6 hours after applying the ventilator. Three patients developed hypothermia 24 hours after treatment, and their blood pressure decreased. In 8 patients, ECMO was established within 24 hours with ECMO adjuvant therapy while administering adrenaline, dopamine, and other drugs. After adjusting various parameters of the ventilator, PaO2, partial pressure of carbon dioxide, and oxygen saturation were significantly improved 1 hour after treatment (Table 3).
| Items | Before | After | t Value | P Value |
|---|---|---|---|---|
| FiO2 | 0.95 ± 0.52 | 0.47 ± 0.08 | 25.67 | 0.000 |
| MAP (cmHg) | 22.38 ± 2.45 | 11.00 ± 2.00 | 37.79 | 0.000 |
| Blood pH | 7.27 ± 0.17 | 7.42 ± 0.05 | -4.19 | 0.001 |
| PCo2 (mmHg) | 70.91 ± 36.73 | 44.39 ± 9.24 | 2.67 | 0.017 |
| PaO2 (mmHg) | 48.94 ± 7.92 | 98.79 ± 44.90 | -4.84 | 0.000 |
| SpO2 (%) | 78.33 ± 6.16 | 96.93 ± 2.77 | -11.89 | 0.000 |
| OI | 44.41 ± 9.03 | 6.32 ± 3.76 | 21.63 | 0.000 |
Abbreviations: MAP, mean airway pressure; PaO2, arterial partial pressure of oxygen; PCo2, partial pressure of carbon dioxide; SpO2, oxygen saturation as measured by pulse oximetry; OI, oxygenation index = (MAP × FiO2 × 100)/PaO2.
After the removal of ECMO, ventilator treatment was continued for 0 to 13 days (4.00 ± 1.00) in all children. In 1 case, the ventilator was used 5 hours after ECMO was removed. The total treatment time of the ventilator was 5 to 52 days, with an average of 17.31 ± 12.45 days.
3.2. Outcomes in Children Treated with Extracorporeal Membrane Oxygenation
Among 16 patients who underwent V-A ECMO mode to establish cardiopulmonary bypass, 15 had successful outcomes and were discharged from the hospital. The offline rate was 100%, and the survival rate was 93.7%. One patient was associated with underlying diseases, such as epilepsy and thrombocytopenia. After 357 hours of ECMO operation, intracranial hemorrhage occurred 4 hours after ECMO was removed and after the lung condition improved. Emergency hematoma evacuation was performed; however, heartbeat respiration occurred due to cerebral palsy, resulting in sudden death. Four patients with brain damage symptoms were transferred to the rehabilitation hospital for further treatment. After 1 month to 3 years of follow-up, the lung function in 15 surviving children returned to normal, including those with brain damage. After 1 to 2 months of rehabilitation, 1 patient was discharged with normal organ functions, as well as good learning and living function (Table 4).
| No. | Gender | Age (y) | Body Weight (kg) | ECMO Establishes a Front Ventilator Usage Time (h) | ECMO Operation Mode | ECMO Operation Hours (h) | No. of Fiberoptic Bronchoscopy-Assisted Treatments (Time) | Days on the Ventilator After ECMO Evacuation (Day) | Total Days on the Ventilator (Day) | Days of Hospitalization (Day) | Results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | 1.1 | 10.00 | 22 | V-A | 210 | 2 | 3 | 13 | 36 | Survival |
| 2 | Female | 4.5 | 18.00 | 2.5 | V-A | 92 | 1 | 3 | 7 | 30 | Survival |
| 3 | Male | 5.3 | 14.30 | 23 | V-A | 192 | 3 | 6 | 15 | 76 | Survival |
| 4 | Male | 0.9 | 9.00 | 108 | V-A | 516 | 6 | 11 | 38 | 58 | Survival |
| 5 | Male | 8.1 | 27.50 | 23 | V-A | 165 | 4 | 0 | 7 | 39 | Survival |
| 6 | Female | 1.4 | 9.00 | 4 | V-A | 121 | 1 | 1 | 6 | 44 | Survival |
| 7 | Female | 4.9 | 18.00 | 46 | V-A | 211 | 3 | 2 | 13 | 73 | Survival |
| 8 | Female | 1.2 | 7.50 | 92 | V-A | 138 | 2 | 9 | 19 | 44 | Survival |
| 9 | Female | 1.9 | 12.00 | 44 | V-A | 304 | 4 | 4 | 19 | 27 | Survival |
| 10 | Male | 0.2 | 6.50 | 214 | V-A | 759 | 10 | 13 | 52 | 106 | Survival |
| 11 | Female | 1.8 | 9.70 | 24 | V-A | 216 | 2 | 2 | 12 | 27 | Survival |
| 12 | Male | 10 | 34.80 | 6 | V-A | 357 | 5 | 1 | 17 | 17 | Offline death |
Abbreviation: ECMO, extracorporeal membrane oxygenation.
3.3. Complications
During the operation of ECMO, 34 complications occurred. Hemorrhage was the most common complication; 6 cases developed intubation bleeding, 1 case gastrointestinal bleeding, 2 cases intracranial hemorrhage, and intracranial hemorrhage occurred in the fifth stage after ECMO in 1 case. One patient developed cardiac arrest and emergency intracranial hematoma evacuation 9 hours after ECMO withdrawal; this child eventually died of cerebral palsy. Hypertension occurred in 7 cases.
Twenty-four hours after establishing ECMO, the blood pressure was lowered using nifedipine and amlodipine besylate tablet; the blood pressure was maintained within the normal range, and the blood pressure returned to normal after ECMO withdrawal. Patients’ complications are shown in Table 5.
| Complication | Incidence (Example, %) |
|---|---|
| ECMO-associated hemolysis | 1 (6.2) |
| Thrombosis | 1 (6.2) |
| Body correlation | - |
| Bleeding | 6 (37.5) |
| Hypocytosis | 2 (7.6) |
| Gastrointestinal bleeding | 1 (6.2) |
| Anemia | 6 (37.5) |
| Pericardial effusion | 4 (25) |
| Abnormal liver function | 6 (37.5) |
| Hypertension | 7 (43.7) |
| Intracranial bleeding | 3 (18.7) |
| Brain injury | 3 (18.7) |
| Bilateral subdural effusion | 1 (6.2) |
| Brain infarction | 1 (6.2) |
| Brain palsy | 1 (6.2) |
| Atrophic brain disease | 6 (37.5) |
| Pancreatitis | 2 (12.5) |
| Abnormal renal function | 1 (6.2) |
4. Discussion
Hypoxemia is a typical clinical manifestation and a leading cause of death in children with ARDS. Although ventilator therapy and some adjuvant treatments may improve ARDS, in the USA, nearly 80% of patients with severe ARDS die each year (6). If conventional treatment is ineffective, ECMO should be considered as early as possible (7). While ECMO is performed at an early stage in the USA, in China, this treatment tends to be delayed. According to the 2015 extracorporeal life support organization (ELSO) data, the global success rate of ECMO treatment in children was 57%, while in China, the rate was only 26% (8). Although the success rate of ECMO treatment improved in 2017 (9, 10), this rate in China is still very low compared to global data. In our study, 16 patients received ECMO; the offline rate was 100%, and the success rate was 93.7%, which is significantly higher compared to previous reports. It is related to appropriate intervention timing, close monitoring of vital signs, and active intervention of complications. On the other hand, it may be a single-center, the number of cases collected is relatively small, and the causes of ADRS during this period are mostly reversible and underrepresented.
There is no uniform defined standard for establishing ECMO in children with respiratory failure. The treatment is usually planned according to the patient’s conditions. A number of studies suggested the use of ECMO before ventilator treatment. Prolonged use of a ventilator before administrating ECMO was associated with more severe lung function damage (11, 12). In children with respiratory failure, mechanical ventilation for more than 2 weeks before ECMO treatment was associated with decreased survival rates (7). The early use of ECMO in children with ARDS can provide support for respiratory function, thus avoiding ventilator-associated lung injury (13). According to the 2009 Multicenter Clinical Study for severe Adult respiratory failure (CESAR) trial performed in the UK, mechanical ventilation time < 7 days was one of the essential criteria when using ECMO treatment (14). In our study, 15 patients received ECMO within 7 days of ventilator treatment, while in only 1 patient, the ECMO treatment was performed later (14 days after the beginning of ventilator treatment). The average ECMO ventilator was 62.3 ± 22.6 hours.
ECMO treatment-related complications are classified into technology-related and organism-related complications. With the application of heparin-coated surface technology, low-resistance polymethyl pentene membrane, centrifugal pump, and other techniques, mechanical-related complications have been reduced. On the other hand, body-related complications, bleeding, infection, renal dysfunction, and nervous system damage are very common after ECMO (15) and related to the continuous use of systemic heparin anticoagulation during treatment. In this study, all patients received intravenous heparin during ECMO. Three patients developed cranial hemorrhage with an incidence of 8.8%, which is in line with the results of Werho et al. who reported an incidence of 3% - 11% (16).
During ECMO treatment, deep sedation is required to prevent bleeding. In our study, 16 patients were treated with midazolam and fentanyl sedation. Three patients with obvious agitation, which affected the ECMO flow, had muscle relaxants. The q1h assessment of pupil size and light reflex is required to closely monitor changes in the nervous system and prevent intracranial hemorrhage. In this study, ACT and coagulation function were monitored every 4 hours, and heparin dosage was timely adjusted; thus, ACT was maintained at 180 ~ 220 seconds. In addition, a computed tomography (CT) scan was performed during treatment and ECMO removal. Two patients underwent a CT scan on the day of ECMO. Intracranial hemorrhage was observed on the head CT of 1 patient 5 hours before ECMO removal. In children with chronic thrombocytopenia, cardiac arrest occurred 9 hours after ECMO withdrawal; bilateral pupils were inconsistent, and hematoma removal was performed at the emergency department after the resuscitation. Eventually, the respiratory heartbeat arrest occurred due to cerebral palsy. During the treatment of ECMO, doctors should pay more attention to the possible occurrence of craniocerebral hemorrhage. Besides, abnormalities of CT and pupil should be timely checked after the end of treatment.
Infants and children have higher neurological complications than adults. Six of the 16 patients in our study were with cerebral atrophic lesions, 3 with brain injury, and 1 case with cerebral infarction. This may be related to the age of the children using VA relevant to ECMO. Children with VV-ECMO have a lower incidence of neurological complications than VA-ECMO, which is not observed in adults (17). It is generally recommended that VV-ECMO be used in children with lung injury without cardiac dysfunction. In the present study, 16 patients received the VA-ECMO mode, and 8 had hemodynamic instability. All the 16 cases were treated with the VA-ECMO mode, which was related to younger age and the lack of suitable type of double-lumen catheterization. When using the VA-ECMO mode, due to the normal heart function of the child, the child was subjected to the blood supply from the ECMO blood return system and the systemic circulation system, and hypertensive symptoms appeared. Among the 16 cases, 7 had hypertension, which was reduced after treatment. With the use of blood pressure drugs, blood pressure returned to normal after ECMO withdrawal. The observation and treatment of hypertension symptoms should also be concerned with ECMO management.
Pancreatitis has not yet been reported after ECMO treatment. In our study, 2 patients developed symptoms of pancreatitis after treatment. One patient developed abdominal pain on the eighth day after the removal of ECMO. In this patient, blood urea gelatinase and lipase were significantly high. After fasting, dehydration, and acid suppression, the patient was on a rice soup diet. Consequently, no abdominal pain or vomiting occurred, and all symptoms improved.
Another patient had blood amylase (421 U/L), blood lipase (918 U/L), and urinary amylase (1533 U/L) on day 9 after ECMO treatment. He had no abdominal pain and vomiting, maintenance of statin, high vein nutrition, acid suppression, and other treatments. On the fifth day of high intravenous nutrition, the nasal jejunal tube was placed for jejunal feeding. The pancreatitis of the child was repeated. After 1 month of treatment, the symptoms of pancreatitis improved. Acute pancreatitis is an inflammatory disease that can be life-threatening if it progresses to severe acute pancreatitis (18, 19). Although the occurrence of pancreatitis is not necessarily related to ECMO treatment, it is necessary to monitor relevant indicators to improve the prognosis in children.
The outcomes of ECMO children were divided into survival rates and related to quality of life. Few long-term follow-up studies have examined ECMO in patients of all ages and with different diseases. VA-ECMO mode treatment usually requires internal carotid artery ligation, which may affect cerebral hypoperfusion and neurological development (20). In our study, 14 children had different degrees of brain damage, including atrophic brain lesions, intracranial hemorrhage, and cerebral infarction. Four of them were transferred to a professional rehabilitation hospital for treatment, and other children were regularly rehabilitated. All patients were followed up for 1, 3, 6 months, and 1 year after the discharge. The follow-up included the recovery of the primary disease, physical fitness, learning ability, nervous system function, etc. After 1 month to 3 years of follow-up, the lung function returned to normal in 15 children, while other organ functions did not show abnormal performance. The learning and living functions were all normal.
The clinical follow-up content and the interval of ECMO postoperative children were not uniform, and the length of follow-up was relatively small. It may be necessary to collect more ECMO follow-up information after clinical use to study the content and time of follow-up. It is important to determine whether ECMO would affect quality of life after surgery.
4.1. Conclusions
Children with ARDS can achieve better clinical results when the ECMO treatment is provided at an early stage. However, ways of preventing complications caused by ECMO should be further investigated by future studies.