1. Background
There are a few methods to secure pulmonary blood flow in duct-dependent cyanotic congenital heart diseases. Prostaglandin E1 modified Blalock Taussig shunt and ductus arteriosus stenting in selected neonates have remarkably improved the prognosis and survival of these patients (1, 2).
According to the Society of Thoracic Surgeons Congenital Heart Surgery Database, the mortality rate for modified Blalock-Taussig shunts is 7.2%, 13.9% in some other reports with more than 17.5% reinterventions (3). The Congenital Heart Surgery Database of the Society of Thoracic Surgeons reported a mortality rate of 7.2% for modified Blalock-Taussig shunts and 13.9% for other reports with more than 17.5% reintervention (3). The mortality rate for surgical shunts has been reported to be approximately 30% in some centers (4). The newer, less invasive approach of patent ductus arteriosus (PDA) stenting to maintain ductal patency might be non-inferior or even superior in comparison to Blalock-Taussig shunts in terms of outcomes and seems to have some advantages over the surgical shunt (5, 6).
Contrary to the place and shape of isolated PDAs, the ductus in cyanotic congenital heart diseases is highly variable. Some of them can arise from the undersurface of the aortic arch or the subclavian artery and may be long, tortuous, or vertical. Some PDAs may be connected to one of the branches of the pulmonary artery with some stenosis, and some may be accompanied by pulmonary artery bifurcation stenosis. It is crucial to consider these variables to perform PDA stenting perfectly.
As a result of the invention of PDA stenting in infants, new technical experiences have been gained (1). The procedure of PDA stenting is technically challenging. Newer techniques and tools, as well as sharing the experiences of operators, may help to improve the outcome of this procedure (1). Some studies described procedural considerations and explained their techniques. The precise attention to detail of these techniques and tips and tricks is critical for ensuring the greater success of ductus arteriosus stenting (1, 7).
This study presents the outcomes of PDA stentings in neonates with pulmonary atresia and ventricular septal defect. We also discussed some details and considerations regarding PDA stenting.
2. Methods
A retrospective study was conducted consecutively on patients with ductal-dependent pulmonary circulation. The PDA stenting was performed during the neonatal period. The inclusion criteria were a SpO2 less than 70% and a Po2 less than 35 torrs before stenting. Exclusion criteria were weight less than 1 kilogram and severe non-cardiac diseases.
The access points, aortic arch-sidedness, size, and brand of the stents were all reported. Each patient's SpO2 and McGoon ratio was determined before stenting and 12 months later.
Some technical considerations for PDA stenting were discussed, including access point, PGE1 infusion, management of pulmonary artery bifurcation stenosis, stent diameter, long and tortuous PDA stenting, thrombosis, and anticoagulation. Angiography and CT scan data have been used to discuss these subjects.
2.1. Procedure Planning
Transthoracic echocardiography was performed according to the guidelines (8, 9) to diagnose the disease, determine the size and confluency of the pulmonary arteries, and evaluate the anatomy of the PDA. Before the procedure, a CT scan was performed if the anatomy of the PDA course and pulmonary arteries was unclear.
The patients received PGE‐1 infusion during admission to prevent PDA constriction before stenting. The patients received PGE‐1 infusion during admission to prevent PDA constriction before stenting. PDA length was evaluated with echocardiography or CT scan. If the PDA was long, we continued the medication until the stent insertion. If it was short, PG E1 was discontinued a few hours before proceeding with the catheterization to prevent stent movement due to excessive dilatation of the PDA.
The most beneficial angiography views to evaluate shape, length, tightness, and the tortuosity of PDAs were lateral and left axial oblique/cranial views for the PDAs originating from the bottom of the aortic arch (10), lateral view for the PDAs originated from the proper place, and anterior-posterior view for the PDAs originated from the subclavian artery. However, some PDAs had bizarre shapes and could not be observed in these views precisely.
Routine hemodynamics measurements, pressure gradients, and arterial saturation were recorded. The access point was mostly from axillary arteries using Judkins's right guiding catheters for the PDAs originated from the aortic arch. Sometimes we reached these PDAs via the antegrade route from the femoral vein through the inferior vena cava, right ventricle, and ventricular septal defect (VSD) to the arch and PDA. The internal mammary guiding catheter was the choice in this approach.
The PDAs originated from the beginning of subclavian arteries and were more accessible from femoral arteries. The stent diameter was chosen as 3.0 - 3.5 mm for less than 3 kg neonates, 3.5 -4.0 mm for 3 - 4 kg neonates, and 4 mm for more than 4 kg neonates (11). If the PDAs were too long, the stent diameter would be increased by 0.5mm (12).
The pulmonary head of the PDA was initially covered, even if we were not successful in covering the entire length. Then, we tried to implant another stent telescopically into the previous one to cover the whole PDA length completely.
Post-procedure care: Heparin with a 100 unit/kg stat dose was started according to the ACT, followed by a 15 - 20 unit/kg/hour infusion dose at least for 24 hours. Five mg/kg aspirin and 0.2 mg/kg clopidogrel were given (11). Clopidogrel was discontinued after a month, but aspirin continued during the follow-up. Five mg/kg/day dipyridamole was given instead of aspirin in patients with glucose-6-phosphate dehydrogenase deficiency. A three-dimensional CT scan of available cases was obtained one year after PDA stenting to realize the need for further procedures. The McGoon ratio of them was compared with the preceding ratio.
The McGoon ratio was calculated by division of the sum of right and left pulmonary arteries diameter at the origin of the upper lobe branch to the descending aorta diameter at the level of the diaphragm in systolic frames (12).
From February 2016 to December 2019, the study was conducted at the Namazi tertiary center of the Shiraz University of Medical Sciences, Shiraz, Iran.
In this study, all procedures were conducted following the ethical standards of the Shiraz University of Medical Sciences research committee and the 1964 Helsinki declaration and its subsequent amendments or comparable ethical standards. The guardians, who were informed about the study, completed an informed consent form. The ethics committee approved this study under the code IR.SUMS.MED.REC.1397.114.
2.2. Statistics
In this study, continuous variables, such as SpO2 and McGoon ratio, are presented as mean ± standard deviation. The Friedman test was used to compare changes in SpO2 and McGoon ratios before the procedure and during the follow-up. Statistical significance was defined as a p-value less than 0.05. IBM SPSS 25 statistical software was used to conduct the analysis.
3. Results
There were 14 females and 12 males among the patients, with a mean age of 17 days. The mean follow-up time was 12 months. There were 19 cases of left-sided aortic arch and 7 cases of the right-sided aortic arch. A total of nine PDAs (34%) originated from the usual location of the aortic arch after the subclavian artery, 12 PDAs (45%) originated from the undersurface of the aortic arch proximal to the subclavian artery, and five PDAs (21%) originated from the commencement of the subclavian artery. These 5 PDAs were long and vertical but not tortuous, while most of the remaining PDAs were tortuous.
Eighteen PDAs (70%) were accessed from the axillary artery, six (23%) from the femoral artery, and two (7%) from the inferior vena cava via the right and left ventricles and ascending aorta.
The PDA stenting procedure was successful in 20 cases (77%) and failed in six cases (23%). Two patients (7.5%) passed away during stenting due to PDA spasm, two cases (7.5%) died the following days due to neonatal sepsis, and two patients (7.5%) underwent surgical shunting.
Two stents were telescopically implanted for three patients, and three telescopic stents were inserted for 2 cases. Three of them (11.5%) underwent other procedures, including modified Blalock-Taussig shunts or stent enlargements before the age of one (Table 1).
Ten families agreed to undergo 3-dimensional CT angiography for their one-year-old children (Table 2). A significant difference was observed between O2 saturation (54.3% ± 7.35 to 77.3% ± 6.55, P-value = 0.032) and McGoon ratio (1.17 ± 0.25 to 1.66 ± 0.89, P-value = 0.041) before and a year after stenting (Table 2).
| No. | Age (mo) | Diagnosis | Aortic Arch | Access Site | Size of Each Stent | Brand of Each Stent |
|---|---|---|---|---|---|---|
| 1 | 23 | Pulmonary atresia | Left | Rt axilla | 3 × 18 | Multilink |
| 2 | 13 | Pulmonary atresia | Left | Rt axilla | 4 × 18 | Cc-flex |
| 3 | 17 | Pulmonary atresia+ DORV | Left | Lt axilla | 4 × 16 | Boston |
| 4 | 9 | Pulmonary atresia | Left | Lt axilla | 4 × 24 | Multilink |
| 5 | 16 | Pulmonary atresia | Left | Rt axilla | 4 × 25 | INVA |
| 6 | 22 | Pulmonary atresia | Right | FA | 4 × 28 | Boston |
| 7 | 9 | Pulmonary atresia | Left | Lt axilla | 3 × 18 | Multilink |
| 8 | 23 | Pulmonary atresia | Right | Lt axilla | 4 × 12 | OMEGA |
| 9 | 8 | Pulmonary atresia | Left | Rt axilla | 4 × 15 | Multilink |
| 10 | 21 | Pulmonary atresia+ DORV | Left | Rt axilla | 4 × 16 | OMEGA |
| 11 | 4 | Pulmonary atresia | Right | FA | 3.5 × 20 | Boston |
| 12 | 7 | Pulmonary atresia | Right | FA | 3.5 × 18 | ORSIRO |
| 13 | 1 | Pulmonary atresia | Left | FA | 3.5 × 24 | OMEGA |
| 14 | 27 | Pulmonary atresia | Left | Lt axilla | 4 × 28 | Boston |
| 15 | 16 | Pulmonary atresia | Left | Rt axilla | 3.5 × 18 | Multilink |
| 16 | 4 | Pulmonary atresia+ DORV | Right | Lt axilla | 3 × 18 | Cc-flex |
| 17 | 6 | Pulmonary atresia | Left | Lt axilla | 4 × 23 | Cc-flex |
| 18 | 1 | Pulmonary atresia | Left | Rt axilla | 4 × 16 | OMEGA |
| 19 | 19 | Pulmonary atresia | Left | Rt axilla | 3.5 × 18 and 4.5 × 16 | Multilink |
| 20 | 20 | Pulmonary atresia | Left | FA | 4 × 16 | Boston |
| 21 | 5 | Pulmonary atresia | Left | FA | 4 × 15 and 4 × 23 | Boston |
| 22 | 11 | Pulmonary atresia | Left | FA | 3.5 × 18 and 3.5 × 15 and 4 × 10 | Multilink |
| 23 | 15 | Pulmonary atresia | Right | Rt axilla | 4 × 23 and 4 × 12 | Multilink |
| 24 | 5 | Pulmonary atresia | Left | Lt axilla | 3.5 × 23 | Multilink |
| 25 | 23 | Pulmonary atresia | Right | Lt axilla | 4.5 × 16 | OMEGA |
| 26 | 11 | Pulmonary atresia | Left | Lt axilla | 4.5 × 16 | OMEGA |
Characteristics of Each Patient and the Size and Brands of the Implanted Stents
| No. | O2 Saturation (%) Before Stenting | O2 Saturation (%) at the Age of One | McGoon Ratio Before Stenting | McGoon Ratio at the Age of One |
|---|---|---|---|---|
| 1 | 60 | 81 | 1.13 | 1.12 |
| 2 | 59 | 79 | 1.12 | 1.77 |
| 3 | 40 | 70 | 1.07 | 1.82 |
| 4 | 57 | 82 | 1.21 | 1.62 |
| 5 | 65 | 89 | 1.12 | 1.28 |
| 6 | 56 | 89 | 1.12 | 2.11 |
| 7 | 60 | 88 | 1.45 | 1.81 |
| 8 | 57 | 87 | 1.12 | 1.82 |
| 9 | 49 | 73 | 0.72 | 1.31 |
| 10 | 40 | 86 | 1.71 | 1.94 |
| Summary of ten cases (mean ± SD) | 54.3% ± 7.35 | 77.3% ± 6.55 | 1.17 ± 0.25 | 1.66 ± 0.89 |
| P-value | 0.032 | 0.041 | ||
O2 Saturation and McGoon Ratio of the 10 Cases Before Stenting and at the Age of One Year
4. Discussion
It is recommended to perform duct-stenting if the procedure is morphologically feasible as a less invasive procedure and a substitute method for surgical shunt (13). Our study found that oxygen saturation was significantly higher at one year of age than during the neonatal period, and McGoon ratios were adequate to permit surgical repair. Thus, PDA stenting results are acceptable up to a year after the procedure (14-20).
Several studies have demonstrated that PDA stenting is less prone to complications than surgical intervention (14-17, 21-23).
However, some studies stated a higher rate of re-intervention compared to surgical shunt due to a gradual decrease in O2 saturation (5, 6, 24).
Our study showed that PDA stenting was not successful in 6 cases (23%); 4 cases (15%) died, two due to PDA spasm during stenting, and two due to sepsis on the following days of stenting. There were fewer deaths associated with our technique than with surgical shunts (4), and some studies reported more or less mortality compared to our study, ranging from 7% to 20% (3, 4, 24).
Nevertheless, PDA stenting may present potential disadvantages, such as stent thrombosis, short stent durability, and a technical impediment to insertion (6).
4.1. Prostaglandin E1
Some studies recommend discontinuing PGE1 a few hours before proceeding to the catheterization to prevent stent displacement due to excessive dilation of PDA (25). In our center, if the PDA was long in echocardiography or CT scan, we continued PGE1, and if it was short, we stopped the medication a few hours before the procedure with standby PGE1 infusion during the operation (1, 19). Because of PDA spasm during PDA manipulation with guide wires, we were unable to perform PDA stenting in 2 cases whose PGE1 had been stopped before the procedure.
4.2. PDA Access
The best access point depends on the location of the PDA. Femoral artery access is suitable for PDAs located in the usual location or intermediate-type PDAs originating from the subclavian arteries (26). According to some studies (6), 73% of these PDAs are approached through the femoral artery. The PDAs originating from underneath the aortic arch can be accessed more easily from axillary arteries, as demonstrated by our approach, which was primarily successful from the axillary arteries (70%). Compared to PDAs with a typical origin, these are usually more complex and have a tortuous pathway, and vascular access from the axillary artery might be prone to more complications (6). For the PDAs originating from the underside of the aortic arch, vascular access anterogradely from the inferior vena cava to the right atrium and the ascending aorta is accessible. It has been performed in our study for 7% of patients successfully, although this access can be risky because of the possibility of conduction disturbances or tricuspid valve damage (7) (Figure 1).
4.3. Bifurcation Stenosis
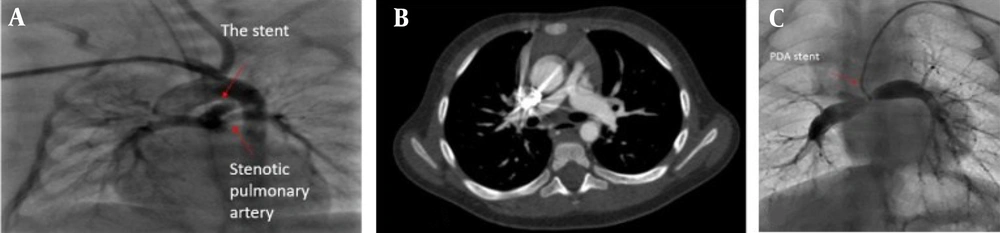
In bifurcation stenosis, PDA stenting is always challenging, and this anatomic variation was observed in 6 cases (23%) of our study. A stent was inserted in the main pulmonary arteries of three patients. The stenosis persisted in only one case; however, the McGoon ratio in all three cases was acceptable for total correction (Figure 2C). If the stent is placed in such a way that enough blood enters the stenotic branch, dilation of the stenosis is possible.
4.4. Unilateral Stenosis of the Right or left Pulmonary Artery Origin
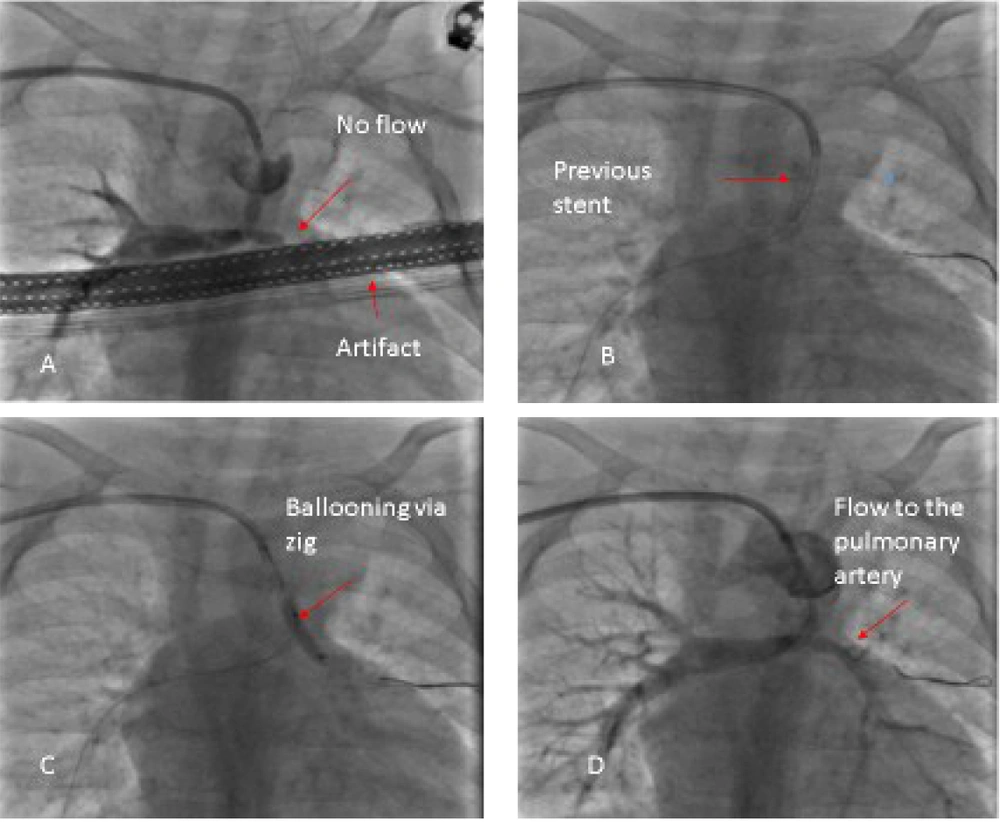
This type of stenosis is considered a class 3 indication for stenting with the C level of evidence; however, some studies have reported successful outcomes of stenting (1). Three of our patients with unilateral pulmonary artery branch stenosis underwent stent insertion in the main pulmonary artery. We passed the wire through stent struts toward the stenotic branch and performed ballooning or stenting of each stenotic region (Figure 3).
A 0.014" guidewire was passed through a strut of the stent toward the stenotic pulmonary artery, and a high-pressure balloon of 2 - 2.5 mm in diameter was passed over it to dilate the struts then another stent was passed through them and inflated.
Our preferred method of steering guidewires through struts was to keep the balloon in the middle of the stent as a holder and booster for the wire so we could guide the wire more easily to the struts. If we suspected stent dislocation due to manipulation, we stopped the procedure, waited for a few months, and then continued further ballooning or stenting.
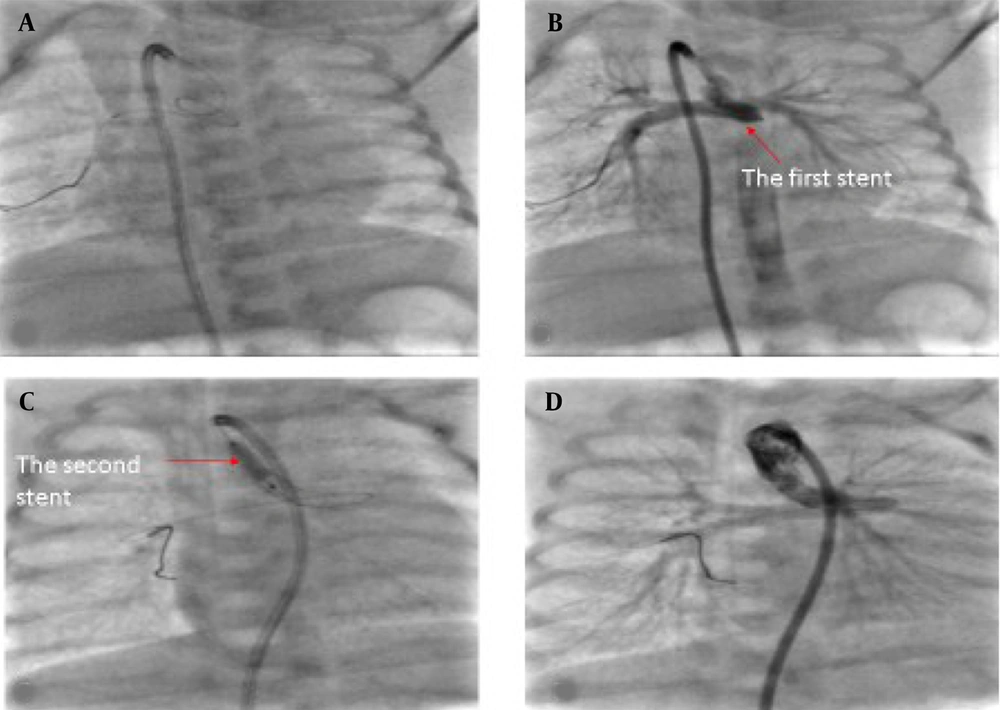
In addition, we inflated the stents into the main pulmonary artery in two cases with unilateral stenosis at the origin of the right or left pulmonary artery. Fortunately, the stenosis resolved during follow-up (Figure 2A and B).
4.5. Stenting of Long Tortuous PDAs
It is preferable to place a stent along the entire length of the duct (25). However, this is not always possible, and it is not uncommon to insert two or more stents telescopically to cover the entire PDA length (13, 27).
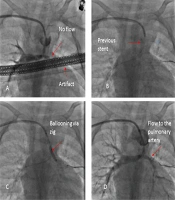
In a long tortuous PDA, we may not be able to pass a long stent through the tortuosity. A short and more flexible stent can initially be passed more easily into the pulmonary head of the PDA, and another stent can be implanted telescopically to cover the entire PDA (Figure 1).
During the procedure, the guide wire should not be pulled back and pulled out of the stent. The second stent could not be inserted otherwise. When the second stent cannot be inserted into the previous stent, we open the second stent near the previous stent, resulting in PDA constriction between the two stents and a decrease in oxygen saturation.
A guidewire is usually inserted into the PDA to guide the stent. Another 0.014 guidewires may be inserted into a PDA to reinforce the strength of the first one and straighten the tortuosity. Moreover, it enables us to steer the stent more easily into the PDA, as we did for seven of our complicated PDAs. Some studies recommend passing a soft coronary guide wire through the tortuous PDA and pulmonary artery with a microcatheter and replacing it with a more stiff guide wire (28).
4.6. Stent Relocation and Displacement
If the opened stent was in a suboptimal position and needed relocation into the PDA, we inflated the stent balloon into the stent again. We changed the stent location carefully and slowly by pulling or pushing it while inflating. If the stent is displaced into the descending aorta, it does not always need to be removed from the aorta or dilated by a balloon. One stent of our patients ran away into the iliac bifurcation; CT angiography two years later showed enough blood flow through and around it, and the iliac artery bifurcation had normal growth (13, 26).
4.7. Stent Diameter
The majority of PDAs that originate from the underside of the aortic arch or the beginning of the subclavian arteries were longer. The use of larger diameter stents was preferred to reduce narrowing since the effects of neointimal proliferation may be greater on these longer PDAs. A larger diameter may, however, result in aortic and coronary blood runoff as well as pulmonary overflow and congestion (29). We also recommend considering the time and the type of surgical repair for choosing the stent diameter. Seemingly, the chance of PDA stenosis increases over time, and a greater stent diameter might reduce this chance. As an example, for a patient who requires homograft at the age of 3 years for a biventricular approach compared to a patient who needs a Glenn operation at the age of 6 months, a greater stent selection is preferred. But, if pulmonary arterial hypertension develops, a larger diameter stent may cause some difficulty for subsequent operations in patients with single ventricular physiology (29).
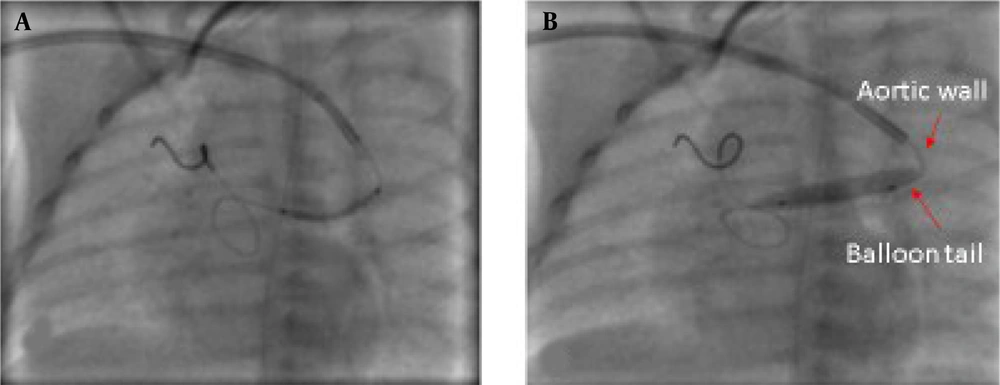
4.8. Considering the Beginning Part of the Balloon, Which is Located Outside of Balloon-Expandable Stents
Balloons are usually longer than stents and PDA stenting is a precise and delicate procedure, and even a few millimeters of the balloon or stents' length can affect the success of the procedure.
The location of the beginning of the balloon is important because the balloon tail may remain in the aorta, and the pressure of this part on the aortic wall during inflation can cause the stent to move forward into the pulmonary artery branches during inflation. On the other hand, the balloon and stent must be completely outside the guiding catheter, and if the stent is outside the catheter and the balloon tail is still inside the catheter, the stent is pushed forward during inflation and placed in an undesirable location (Figure 4).
4.9. Durability and Thrombosis
Heparin fluid flush should be used during surgery, and bolus doses of heparin should be injected as a thromboprophylaxis agent. Accurate measurement of the dose of heparin used for the baby is crucial to prevent stent thrombosis and catastrophic bleeding. The bolus dose of heparin should be adjusted according to the weight of the baby and the dose of heparin in the fluid flush to prevent troublesome bleeding or thrombosis. A helpful guide is the activated clotting time.
During follow-up, neointimal proliferation was not observed with our drug-eluting stents, whereas stents without drug-eluting properties may result in neointimal proliferation and thrombosis (26).
5. Conclusions
PDA stenting appears to be a less invasive method of securing pulmonary blood flow in patients with pulmonary atresia and ventricular septal defect. There is a need for further research to determine PDA-specific anatomic characteristics that would benefit from stenting, and to determine the challenges related to the PDA stenting procedure, as this technique is not straightforward, especially in long and tortuous PDAs.