1. Context
Children admitted to the intensive care unit are at risk of malnutrition, mainly due to chronic diseases they are suffering from. These patients require a different nutritional diet regimen from those in a normal or stable disease state due to change in metabolism under the stress of diseases. The research on how pediatric intensivists should manage such patients in different states of disease is ongoing, and there are some important questions that have no definitive answers due to lack of evidence. In Iran, at present, we have 19 pediatric intensive care units with 400 beds. At some of these centers, there are registered dietitians with experience and knowledge for managing nutrition problems. However, for the correction and unification of treatment protocols, a consensus based on current evidence is necessary. The goal of nutrition therapy during critical illness is to meet the patient’s basal metabolic needs, support the body in response to stress and illness, and prevent the ongoing loss of lean body mass (1, 2). This Nutrition section position statement provides and discusses the important nutrition instructions for pediatric intensivists, pediatricians, registered dietitians, and nurses who take care of infants more than one month old, children, and adolescents to optimize nutrition in critically ill children. This was to describe compliance with current recommendations and guidelines and to compare knowledge between professional groups. The results of this study will serve as a base for future educational interventions.
2. Methods
The study was performed in three different parts. According to the Scottish Intercollegiate Guidelines Network (SIGN) guideline based on evidence, first, articles matching our criteria were extracted from the literature, and then the strength of evidence was evaluated. The SIGN published an evidence-based guideline on the condition in November 2006. The guideline’s purpose is to avoiding unnecessary tests and interventions as well as decrease some of the reported variations in management. It covers prevention; recognition and differential diagnosis; indications for hospital admission and the in-patient management of infants with bronchiolitis; limiting disease transmission, and prognosis. Its scope performed for infants up to12 months of age and excludes management in intensive care. The other recommendations in the guideline can readily be fined in the SIGN website https://www.sign.ac.uk/).
The systematic literature search was performed in four databases Embase, Medline, Cochrane, Web of Science and included all articles published from 1997 until May 2020. The search terms used per question described in Appendix 1 (see Supplementary File). Our Inclusion criteria were RCTs, case–control, before and after and cohort studies including critically ill term neonates and children (aged ≥ 37 weeks’ gestational age—18 years). We excluded publications describing studies in pre-term infants, unless the question specifically related to neonatal PICU patients and no evidence existed in term neonates. Also, animal studies, case reports, editorials, commentaries, conference abstracts and letters were excluded. The initial database search found 2860 articles; after duplicates were eliminated (1250), 1610 articles remained. After reviewing across the titles and abstracts, 1606 papers were outputted for full-text examination. Finally, 4 articles were included in the recommendation synthesis (3-6).
Based on the results from the advanced search, a first draft of recommendations was composed, including the supporting text and grade of recommendation. The strength was measured on a scale of 1 - 4: (1) high, (2) moderate, (3) low, and (4) very low. In addition, the recommendation level was marked A, B, C, and D according to the according to the SIGN grading system (7). Finally, a summary of statements consisting of details regarding the strength of evidence and recommendation level was reviewed by 12 experts, and two-round surveys were accomplished according to the Delphi method to reach a consensus. In the first round, 27 statements in 5 categories were sent to 6 pediatric and adult pulmonologists and intensivists, 4 pediatric gastroenterologists, 1 clinical pharmacist, and 1 pediatric nutritionist. All experts had at least 5 years of experience in their respective fields. They commented on the statements using one of the following: S (strong), W (weak), the F (further research needed). In the second round, the opinions of all colleagues were summarized and sent back to the experts for review.
Any recommendations were discussed with an agreement equal to or less than 95%. Finally, the summary of statements with strength of evidence, grade of recommendations, and expert opinions are given in Table 1 (8). We believe taking the best decision about the management of patients should be individualized, and there are a lot of conditions like inborn metabolic disease that these statements are not applicable to.
| Statement | Strength of Evidence | Recommendation Level | Expert Opinion |
|---|---|---|---|
| Nutritional assessment | |||
| 1. Nutritional assessment of children should be performed immediately on arrival at the intensive care unit so malnourished patients and those at risk of malnutrition can be identified. | Low | A | S |
| 2. Anthropometric studies are the screening method of choice to asses admitted children in intensive care unit. | Low | A | S |
| 3. Lab test like albumin, prealbumin, thyroid binding globulin are not suitable tests for evaluation of nutritional status of children admitted in intensive care unit. | Low | B | S |
| 4. It seems that new procedures at the bedside, which are able to measure body composition parameters such as lean body mass (the amount of lean body weight without fat), the amount of body fat and the total body water, are needed. | Low | B | F |
| Calculation of energy consumption | |||
| 1. The best method to evaluate the energy consumption is the indirect method of measuring calories (indirect calorimetry). | Moderate | A | S |
| 2. If indirect calorimetry is not available Schofield or WHO equation are used to calculate the energy. | Low | B | S |
| 3. It is not commonly suggested to use the activity and stress factors when calculating the energy for patients in intensive care unit. | Moderate | C | F |
| 4. Experts recommend provide at least 67% of calories need through the end of first week. | Low | C | F |
| 5. Some valuable research offer 54 - 58 kcal/kg/d is minimum need for protein balance and prevention from catabolism. | Moderate | B | F |
| Choosing the method of feeding | |||
| 1. Enteral feeding is the best route of nutrition if it is feasible. | Moderate | A | S |
| 2. The best time of initiation of enteral feeding is first 24 - 48 hours of admission. | Low | B | S |
| 3. Achieving the nutrition/calorie goal is possible if there is written protocol in intensive care unit. | Low | C | W |
| 4. The presence of a team of nutrition specialists helps to improve the nutritional status in the PICU. | Low | C | S |
| 5. Gastric feeding is more practical if it is feasible. | Moderate | B | S |
| 6. Bolus or continuous feeding has the same efficacy and there is not enough evidence to prove advantage of one. | Low | C | F |
| 7. Gastric residual volume is not measured as a marker of feeding intolerance. | Low | C | F |
| Feeding goals | |||
| 1. In critically ill patients to create a positive protein balance at least 1.5 grams of protein per weight per day is needed. | Moderate | A | S |
| 2. Polymeric enteral formula is the first choice in patients admitted in intensive care unit. | Low | B | F |
| 3. Amino acid based formula should be considered in condition of moderate to severe food allergy. | Moderate | B | F |
| 4. Peptide formula may be indicated in patients who are intolerant to polypeptide formula, small intestine absorption capability is reduced or gastric emptying is delayed. | Low | B | F |
| 5. Routine administration of glutamine, arginin or micronutrients is not indicated. | Moderate | B | S |
| Parenteral nutrition | |||
| 1. Parenteral nutrition is indicated in patients enteral nutrition is absolutely contraindicated. | Moderate | B | S |
| 2. Parenteral nutrition is not indicated in first 24 of admission in any children. | Moderate | A | S |
| 3. The best time or content of parenteral nutrition suitable for infants or children is not well defined. | Low | B | S |
| 4. Parenteral nutrition is not recommended in children who start enteral feeding in a few days from NPO time. | Low | C | F |
| 5. Supplementary parenteral nutrition is not recommended to achieve more calorie goal in the first week of admission instead of exceptional condition like severe malnutrition. | Low | B | F |
| 6. Glucose infusion should start early if indicated and the infusion rate should be monitored. Hypoglycemia or hyperglycemia must be prevented. | Low | A | S |
Abbreviations: PICU, pediatric intensive care units; NOP, nil per os; S, strong; W, weak; the F, further research needed.
3. Results and Discussion
A summary of all recommendations is shown in Table 1 (8, 9).
3.1. Nutritional Assessment
(1) Nutritional assessment of children should be performed immediately on arrival at the intensive care unit so malnourished patients and those at risk of malnutrition can be identified.
(2) Anthropometric studies are the screening method of choice to assess children admitted to the intensive care unit.
(3) Lab tests like albumin, prealbumin, and thyroid binding globulin are not suitable for the evaluation of the nutritional status of children admitted to the intensive care unit.
(4) New procedures at the bedside, which are able to measure body composition parameters such as lean body mass (the amount of lean body weight without fat), the amount of body fat, and the total body water, are needed. Negative protein balance may result in loss of lean body mass (LBM), which has been associated with poor outcomes in critically ill patients. Quantifying the amount of LBM and fat mass upon admission offers a valuable addition to tailor early nutritional interventions. In this regard, quantifying LBM may be especially helpful in guiding protein dosing, as LBM contains the body’s largest protein store. Looking beyond the scope of nutritional support, quantifying LBM and fat mass might be helpful in dosing of other medication, and provide information on preadmission status, possibly with important consequences for decisions regarding treatment options and treatment limitations. Also, the use of percentiles and height-normalized LBM and body fat permit the classification of patients as under or over nourished (10-12).
Anthropometric studies in patients admitted to the intensive care unit are considered one of the best methods of assessment (6, 13-15). Evaluation of the nutritional status of children is performed by calculating the Z score based on the World Health Organization (WHO) recommendations or the Centers for Disease Control and Prevention (CDC) guidelines, which includes weight versus age, weight versus height, head circumference (for children younger than 2 years), body mass index (for children over 2 years). The severity and chronicity of disease should be well defined, and then patients who are malnourished or at risk of malnutrition recognized (15-19). Anthropometric measurements are performed in the first 24 hours of hospitalization and are repeated at least once every week (6, 13-15). Weighing is one of the most valuable methods for assessing the nutritional status of patients admitted to the intensive care unit, but it is not always possible to carry this out for all patients. Beds equipped with a weight gauge might be used for infants (20, 21). Anthropometric studies in patients admitted to the intensive care unit might have low accuracy and sensitivity due to the impact of underlying diseases, such as trauma (water retention and swelling) (5). An alternative procedure to study malnutrition is using the mid upper arm circumference measurement, which gives closest results compared to the body weight (22). This method of assessment is more accurate in detecting acute malnutrition compared to weight for height measurement in the presence of water retention and edema (23). The mid upper arm circumference measurement is an independent method to determine the nutritional status of 6- to 59-month-old children (24). The table showing arm circumference standards relative to age has been published by WHO (Appendix 2 in Supplementary File) (19). Serial measurement of mid upper arm circumference is a reliable method for assessing hospitalized patients and is more accurate than measuring the weight relative to height in predicting mortality in children with acute malnutrition who are admitted to intensive care units (25, 26).
| Classification | Variable | Grade/ Definition |
|---|---|---|
| Gomez et al. (27) | Median WFA (%) | Mild (grade 1): 75 - 90% WFA; Moderate (grade 2): 60 - 74% WFA; Severe (grade 3): < 60% WFA |
| WHO (wasting) | WFH (Z scores below median WFH) | Moderate: Z score between -2 and -3; Severe: Z score < -3 |
| Cole et al. (16) | BMI (BMI Z scores for age) | Grade 1: Z scores for age < -1; Grade 2: Z scores for age < -2; Grade 3: Z scores for age < -3 |
Abbreviations: WFA, weight-for-age; BMI, body mass index.
Length is obtained supine in children younger than 2 years or in older children unable to stand. Alternative measures such as upper arm length or lower leg length may be obtained to estimate body length in children who have contractures or scoliosis. Reference standards are available for upper arm length and lower leg length in children 2 years and older. Standing height, without shoes and braces, is recorded in all other children. Weight is measured on the same scale with the child wearing little or no clothing. Children with severe disabilities may be weighed while being held by a parent or while seated in a wheelchair. BMI can be calculated from height and weight measurements of children 2 years and older. Although the inability to measure standing height theoretically invalidates the calculation of BMI, estimates derived from lower leg length serve as a practical alternative in the clinical setting. Since formal methods of determining nutritional status in children are not accurate in situations such as fluid retention and edema, more appropriate methods such as body composition assessment are required. However, currently, there is no other method except DEXA scanning, which is not useful in the absence of clear standards for children's age. New procedures at the bedside, which are able to measure parameters such as lean body mass, the amount of body fat, and the total body water, are needed (28).
3.2. Calculation of Energy Consumption
(1) The best method to evaluate energy consumption is the indirect method of measuring calories (indirect calorimetry).
(2) If indirect calorimetry is not available, Schofield or WHO equations can be used to calculate energy consumption.
(3) It is not commonly suggested to use the activity and stress factors when calculating energy consumption in patients admitted to the intensive care unit.
(4) Experts recommend the need for at least 67% of calories through the end of the first week.
(5) Some valuable research offers a minimum requirement of 54 – 58 kcal/kg/day for protein balance and the prevention of catabolism.
The most appropriate method for evaluating energy consumption is the indirect method of measuring calories (indirect calorimetry). Indirect calorimetry is a reliable, no-risk, non-invasive, and reproducible method. Besides, it is possible to use this method when the patient is connected to a ventilator. Measurement of energy consumption in these critically ill children can help determine the status of the child, whether in hyper- or hypo-metabolic state, which is achieved by using indirect calorimetry on a daily basis. The indirect calorimetry method has its limitations. For example, pain and fever and the use of medicines have an impact on the calculation of energy consumption using indirect calorimetry, which is normally ignored when using this method (29-31). Using formulas designed for measuring resting energy expenditure is not a reliable method of assessing children in the intensive care unit. If indirect calorimetry is not available, then Schofield or WHO equations can be used to calculate the energy level (Table 3) (32-34). It is not recommended to use the activity and stress factors when calculating the energy in patients in intensive care unit (6, 13-15). Provision of a sufficient amount of calorie is important for maintaining the health of children, and experts recommend the need of at least 67% of calories through the end of the first week. Some valuable research offers a minimum requirement of 54 - 58 kcal/kg/day for maintaining protein balance and prevention of catabolism (6, 13-15, 35, 36).
| Age (y) | Sex | Schofield | World Health Organization |
|---|---|---|---|
| 0 - 3 | M | 0.167W+ 15.174H - 617.6 | 60.9W -54 |
| 0 - 3 | F | 16.252W+10.232H - 413.5 | 61W - 51 |
| 3 - 10 | M | 19.59W+1.303H+414.9 | 22.7W+495 |
| 3 - 10 | F | 16.969W+1.618H+371.2 | 22.5W+499 |
| 10 - 18 | M | 16.25W+1.372H+515.5 | 17.5W+651 |
| 10 - 18 | F | 8.365W+4.65H+200.0 | 12.2W+746 |
| Respiratory quotients (RQ) a | |||
| VO2 = VI(FIO2)-VE(FEO2) | |||
| VCO2 = VI(FICO2)-VE(FECO2) | |||
| RQ = VCO2/VO2 | |||
| Fuels respiratory | Quotients | ||
| Carbohydrate | RQ = 1 | ||
| Fat | RQ = 0.7 | ||
| Protein | RQ = 0.8 | ||
| REE can be derived from metabolic cart measurements, using the following formulae: | |||
| REE = 5.68 VO2 + 1.59 VCO2 -2.17 Urine N2 | |||
| If urine N2 is not entered, the REE is calculated as follows: | |||
| REE = 5.466 VO2 + 1.748 VCO2 | |||
a According to Haldane transform, which states the relationship between the inspiratory (I) and expiratory (E) volume, the respiratory quotients can be calculated from gas fractions alone.
3.3. Choosing the Method of Feeding
(1) Enteral feeding, if feasible, is the best route of nutrition. Meal observation may be useful because of the variable feeding patterns in neurologically impaired children.
(2) The best time of initiation of enteral feeding is the first 24–48 hours of admission.
(3) There should be a clearly-defined protocol on achieving the nutrition/calorie goal in the intensive care unit.
(4) Registered dietitians who have enough experience of managing nutrition challenges manages the nutritional status of patients in the intensive care unit more efficiently.
(5) Gastric feeding, if feasible, is more practical.
(6) Bolus or continuous feeding has the same efficacy, and there is not enough evidence to prove the advantage of one above the other.
(7) Gastric residual volume is not measured as a marker of feeding intolerance.
Unless there is no absolute/relative contraindication such as intestinal surgery or obstruction, hemodynamic instability, and severe gastric or intestinal dysmotility with recurrent vomiting, enteral nutrition is the most appropriate method of feeding (6, 8, 13-15, 18). Compared with parenteral feeding, the enteral approach has some benefits, including protection of the gastrointestinal tract, ease of use, safety, no side effects such as infections caused by catheters or liver diseases, as well as two to four times lower cost (8). The best time of initiation of enteral feeding is the first 24 - 48 hours of admission (6, 13-15). Some experts recommend earlier enteral feeding (within 6 hours of admission), if possible, and not as late as 48 hours from admission. And even better would be achieving at least one-fourth of the nutrition goal in the first 48 hours (37-42). There are several methods of feeding patients in the pediatric intensive care unit (Appendix 3 in Supplementary File) (43-47). Some studies show that the presence of a team of nutrition specialists helps improve the nutritional status of patients in the pediatric intensive care unit (6, 15). The most common reasons for not delivering enough energy to the patients include clinical instability, difficulty in breathing, diagnostic procedures, gastrointestinal complications, and the use of drugs (34). While selecting the most appropriate route of feeding (gastric versus post-pyloric or small intestine), health of the digestive system, feeding duration, and the risk of aspiration should be taken into consideration (34). Overall, gastric feeding is more practical. Several studies have compared continuous feeding (infusion) versus intermittent feeding (bolus). No significant differences between the two methods in terms of their tolerance and side effects have been reported (48, 49). Gastric residual volume is not measured as a marker of feeding intolerance. There are insufficient data to conclude that gastric residual volume is related to aspiration. Vomiting is probably a better maker for making a decision about the ways of advancing the volume of enteral feeding (49-52). Percutaneous endoscopic gastrostomy (PEG) placement, a minimally invasive non-surgical procedure, involves little discomfort; and the feeding device can be used within a few hours of installation. The child with symptoms suggestive of chronic aspiration may require a chest x-ray and an evaluation by a pulmonologist, especially if surgical intervention for enteral access is considered. Monitoring O2 saturation during a meal may be important because ill children may have hypoxemia while being fed some food textures. Measured resting energy expenditure (REE) and Respiratory Quotients (RQ) indicated in Table 3 (4) .
3.4. Feeding Goals
(1) In critically ill patients, the intake of 1.5 g/kg/day or higher to prevent cumulative negative protein balance day is needed.
(2) Polymeric enteral formula is the first choice in patients admitted to the intensive care unit. Nutritionally complete formulas for oral supplementation or tube feeding are available for a variety of ages and conditions (Table 4) (53)
| Formula | Caloric Density (kcal/mL) | Carbohydrate | Protein | Fat | Indications |
|---|---|---|---|---|---|
| Ensure | 1.06 | Sucrose, corn syrup, corn maltodextrin | Milk protein concentrate, soy protein concentrate | Soy, canola, and corn oils | Complete oral/enteral supplement |
| Pediasure | 1.0 | Sucrose, corn maltodextrin | Milk protein concentrate, whey protein concentrate, soy protein isolate | High oleic safflower, soy, and medium chain triglyceride oils | Complete feeding for 1 - 13 y |
| EleCare | 1.0 | Corn syrup solids | L-amino acids | soy, oleic acid, safflower oils | Free amino acid feeding for 1 - 10 years |
| Peptamen junior | 1.0 | Maltodextrin, corn starch | Enzymatically hydrolyzed whey protein | Medium chain triglyceride, soy, and canola oils | Peptide based feeding for 1 - 10 y |
| Neocate | 1.0 (standard dilution) | Corn syrup solids | 100% free amino acids | Fractionated coconut, canola, and high oleic safflower oils | Hypoallergenic feeding for 1 - 10 y |
(3) Amino acid based formula should be considered under conditions of moderate to severe food allergy.
(4) Peptide formula may be indicated in patients who are intolerant to the polypeptide formula, if small intestine absorption capability is reduced or gastric emptying is delayed.
(5) Routine administration of glutamine, arginine, or micronutrients is not indicated.
In severe diseases caused by surgery or trauma, protein catabolism dramatically increases and the change of muscle protein into glucose is an excellent adaptive response for a short time. However, since this source of protein is limited in children and infants, it is not appropriate for long periods. Unlike fasting (starvation), providing the carbohydrate alone is not a good method of reducing glucose production via gluconeogenesis (34). So, without addressing the cause of catabolism (illness or injury), progressive destruction of muscle proteins leads to the loss of diaphragmatic, intercostal respiratory and cardiac muscles. Giving enough protein can improve wound healing and inflammatory response and maintenance of the skeletal muscles. However, giving extra protein is likely to cause toxicity, especially in patients with liver and kidney diseases (54). In some studies on critically ill patients, it has been observed that to create a positive protein balance at least 1.5 g of protein per weight per day is needed (6, 13-15, 55-57). There is not enough evidence to recommend more protein in a setting of acute critical illness. The optimum protein need for different situations is not well defined and the recommended dietary allowance of protein recommended by WHO for normal children is not applicable for children admitted to the intensive care unit (40, 58-61). Routine administration of glutamine, arginine, or micronutrients is not indicated (62).
Glycogen storage in children is limited and is diminished rapidly in disease and injury. This is the reason muscle protein catabolism is activated to produce glucose in pediatric patients. Hyperglycemia in critically ill patients is extremely common, and in addition, it is a sign of worsening prognosis (63, 64). At present, there is no study that has confirmed the importance of intensive glycemic control in critically ill patients, but hypoglycemia and hyperglycemia are both related to prolonged stay of patients in intensive care units. Currently, exact blood glucose control in these children remains undefined (65).
Critically ill children are at risk of reduced levels of micronutrients and antioxidants. This will disrupt the metabolic system performance. Further studies are needed to confirm whether the use of micronutrients has any positive effect on the outcome in these children. At present, measurement and supplementation of micronutrients in cases of shortage is recommended for those critically ill patients who are hospitalized for extended periods (10 - 14 days). This is important especially in the case of children who are on dialysis (66).
3.5. Parenteral Nutrition
(1) Parenteral nutrition is indicated in patients in whom enteral nutrition is absolutely contraindicated.
(2) Parenteral nutrition is not indicated in the first 24 hours of admission in children.
(3) The most appropriate time or amount of parenteral nutrition suitable for infants or children is not well defined.
(4) Parenteral nutrition is not recommended in children who start enteral feeding within a few days of NPO.
(5) Supplementary parenteral nutrition is not recommended to achieve the more calorie goal in the first week of admission, except in conditions like severe malnutrition.
(6) Glucose infusion should start early if indicated and the infusion rate should be monitored. Hypoglycemia or hyperglycemia must be prevented.
Parenteral nutrition is indicated in circumstances where enteral nutrition is absolutely contraindicated. Parenteral nutrition is not indicated in the first 24 hours of admission in children. The best time or content of parenteral nutrition suitable for infants or children is not well defined. Parenteral nutrition is not recommended in children who start enteral feeding in a few days from NPO time otherwise parenteral nutrition might be considered. Supplementary parenteral nutrition is not recommended to achieve more calorie goal in the first week of admission instead of exceptional condition like severe malnutrition or intestinal failure (6, 13-15, 67).
Energy requirements may be 5 – 10% lower when energy is supplied through parenteral nutrition (68). Carbohydrates contribute 50 – 60% of the calorie requirement, fat 10 - 25%, and protein 25 - 35%. Monitoring of growth parameters must be done to prevent complications out of excess energy like immune deficiency, fatty liver, azotemia, and hyperglycemia (69). In obese children, energy requirement is the same as in others (70).
There is no sufficient evidence available to conclude what type of parenteral amino acid should be the one of choice. Energy is provided at a rate of 4 kcal/g. Protein needs of healthy children are not sufficient to meet the needs of acutely ill children. The protein needs of ill children falling in different age groups have been proposed by ASPEN (Appendix 4 in Supplementary File) (18). There are different opinions about the calculation of calories from protein required in acutely ill children. Positive nitrogen balance is more attainable by enteral protein compared to parenteral protein. Calorie-to-protein ratio of 130–150 kcal/g nitrogen is recommended (15).
Carbohydrates are usually intravenous dextrose solutions, and each gram supplies 3.4 kcal of energy. Glucose infusion should start early if indicated and the infusion rate should be monitored. Hypoglycemia or hyperglycemia must be prevented. Glucose infusion rate (GIR) should be measured. Urine glucose is one of the best ways of monitoring hyperglycemia. Glucosuria should be double checked by serum glucose. The appropriate GIR related to age is shown in Appendix 4 (see Supplementary File) (71).
There are different types of fat suitable for intravenous infusion. Fat containing long-chain triglyceride from soy is a well-known intralipid, a 20% intravenous fat emulsion, commonly used in children older than 6 months (72, 73). Omegaven (pure fish oil) for infants under 6 months old or in conditions like parenteral nutrition associated liver disease (PNALD) is the treatment of choice (74). Emulsion with different sources of lipid like SMOFlipid is approved for use in Europe. SMOFlipid can be used in PNALD (75-79). The dosage for different ages can be found in Appendix 4 (see Supplementary File) (78, 79). SMOFlipid contains a mixture of 4 different lipid sources: soybean oil providing essential fatty acids, olive oil rich in monounsaturated fatty acids which are less susceptible to lipid peroxidation than polyunsaturated fatty acids, medium-chain triglycerides showing a faster metabolic clearance than long-chain triglycerides, and fish oil for the supply of omega-3 fatty acids.
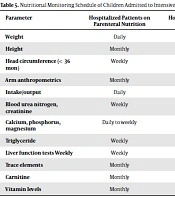
Although the weight of evidence still seems to support a role for PN in selected pediatric patients admitted to intensive care unit, close monitoring of laboratory values and clinical condition in addition to the specifics of the PN prescription are necessary to prevent complications (Table 5) (9).
| Parameter | Hospitalized Patients on Parenteral Nutrition | Hospitalized Patients on Oral/Tube Feedings | Outpatients on Parenteral Nutrition | Outpatients on Oral/Tube Feedings |
|---|---|---|---|---|
| Weight | Daily | Daily | Weekly | Monthly |
| Height | Monthly | Monthly | Monthly | Monthly |
| Head circumference (< 36 mon) | Weekly | Weekly | Monthly | Monthly |
| Arm anthropometrics | Monthly | As indicated | Monthly | As indicated |
| Intake/output | Daily | Daily | Daily to weekly | Weekly to monthly |
| Blood urea nitrogen, creatinine | Weekly | Weekly | Weekly | Monthly |
| Calcium, phosphorus, magnesium | Daily to weekly | Weekly | Weekly | Monthly |
| Triglyceride | Weekly | Monthly | Weekly | As indicated |
| Liver function tests Weekly | Weekly | Weekly | Monthly | Monthly |
| Trace elements | Monthly | As indicated | Biannually | As indicated |
| Carnitine | Monthly | As indicated | Biannually | As indicated |
| Vitamin levels | Monthly | As indicated | Biannually | As indicated |
3.6. Treatment Team of Pediatric ICU Specialists
A treatment team of pediatric ICU specialists should include the following (80-82):
3.6.1. Physician Staff
Studies suggest that having a full-time pediatric intensivist in the PICU improves patient care and efficiency (4-8). At certain times of the day, the attending physician in the PICU may delegate the care of patients to a physician of at least the postgraduate year 2 level (in a level I PICU, this physician must be assigned to the PICU, and in a level II PICU, this physician must be available to the PICU) or to an advanced practice nurse or physician’s assistant with specialized training in pediatric critical care. These non-physician providers must receive credentials and privileges to provide care in the PICU only under the direction of the attending physician, and the credentialing process must be made in writing and approved by the medical director. An in house physician at the postgraduate year 3 level or above in pediatrics or anesthesiology is essential for all level I PICUs. In addition, all hospitals with PICUs must have a physician in-house 24 hours per day who is available to provide bedside care to patients in the PICU. This physician must be skilled in and have credentials to provide emergency care to critically ill children.
Depending on the unit size and patient population, more physicians at higher training levels may be required. Other physicians, including the attending physician or his or her designee, should be available within 30 minutes to assist with patient management. For level I units, available physicians must include a pediatric intensivist, a pediatric anesthesiologist, a pediatric cardiologist, a pediatric neurologist, a pediatric radiologist, a psychiatrist or psychologist, a pediatric surgeon, a pediatric neurosurgeon, an otolaryngologist (pediatric subspecialist desired), an orthopedic surgeon (pediatric subspecialist desired), and a cardiothoracic surgeon (pediatric subspecialist desired). For level II PICUs, pediatric subspecialists (with the exception of the pediatric intensivist) are not essential but are desirable, a general surgeon and neurosurgeon are essential, and an otolaryngologist and orthopedic surgeon are desirable (pediatric subspecialists optional). For level II PICUs, a cardiovascular surgeon is also optional. For level I PICUs, it is desirable to have available on short notice a craniofacial (plastic) surgeon, an oral surgeon, a pediatric pulmonologist, a pediatric hematologist/oncologist, a pediatric endocrinologist, a pediatric gastroenterologist, and a pediatric allergist or immunologist. These physicians should be available for patients in level II PICUs within a 24-hour period.
3.6.2. Respiratory Therapy Staff
The respiratory therapy department should have a supervisor responsible for performance and training of staff, maintaining equipment, and monitoring multidisciplinary quality improvement and review. Under the supervisor’s direction, respiratory therapy staff primarily designated and assigned to the level I PICU shall be in-house 24 hours per day. Hospitals with level II PICUs must have respiratory therapy staff in-house at all times; however, this staff need not be dedicated to the PICU (unless patient acuity so dictates). All respiratory therapists who care for children in level I and II PICUs should have clinical experience managing pediatric respiratory failure and pediatric mechanical ventilators and should have training in PALS or an equivalent course.
3.6.3. Ancillary Support Personnel
An appropriately trained and qualified clinical pharmacist should be assigned to the level I PICU; this is desirable for the level II PICU. Staff pharmacists must be in-house 24 hours per day in hospitals with level I PICUs, and this is desirable in hospitals with level II PICUs. Biomedical technicians must be available within 1 hour, 24 hours per day for level I and II PICUs. For level I PICUs, unit secretaries (clerks) should have primary assignment in the PICU 24 hours per day. A radiology technician (preferably with advanced pediatric training) must be in-house 24 hours per day in hospitals with level I PICUs, and this is strongly recommended for those with level II units. In addition, social workers; physical, occupational, and speech therapists; nutritionists; child life specialists; clinical psychologists; and clergy must be available (this is essential for level I and desirable for level II PICUs).
This study has some limitations. We acknowledge that the recommendation is intended for term neonates, so it is less useful for pre-term infants. This is due to the complexity of clinical care in these infants, which requires further study. Unfortunately some of their recommendations are not practical in our PICUs due to lack of facilities. However, we tried to modify some of the anthropometric measurements according to the available possibilities.
4. Conclusions
Nutrition management of patients admitted to the pediatric intensive care unit is an important step towards maintaining the health condition of children. Prevention of infection, normal growth and development, and rapid wound healing are some of the benefits of proper nutrition management. Rapid nutritional assessment, judging patients with malnutrition or at risk of malnutrition, fast intervention with early enteral nutrition, reaching the protein and energy goals under the supervision of an expert registered dietitian, and persistent monitoring with minimizing the time of fasting are some of the key components of proper nutrition management based on evidence found in the literature. There are still questions open about the exact protein need in different underlying diseases, best time of initiation of parenteral nutrition alone or in combination with enteral nutrition, best enteral formula, and many other vital points that are essential to better management of critical ill patients.