1. Background
There is a relative lack of information about the consequences/complications of antibiotic therapy during pregnancy or the first postnatal days on brain development, function, and subsequent issues. This is probably because these drugs have long been considered safe (1). Antibiotics can exert some effects not only on the pathogens but also on the natural host microbiome (microbiome of the skin and oral mucosa, gastrointestinal tract, and vagina) (2). Numerous animal studies have shown that the enteric microbiome influences the physiology and evolution of organs other than the host gastrointestinal tract. These findings raise the possibility that changes in the developing microbiome of the neonate's intestine may directly or indirectly alter the developmental pathways of the brain and affect its subsequent function in life (3). Nevertheless, some studies have indicated that a healthy, natural gastrointestinal microbiome can play a key role in the proper development of the nervous system (4). The highly rapid production of synaptic connections reaches its peak between 3 and 24 months, depending on the area of the cerebral cortex, and interestingly, the maturation of the intestinal microbiome also occurs during the first two to three years of life after birth, coinciding with an important stage of the primary cerebral growth (1). Successful and effective feeding is a complex process that requires the coordination of the swallowing, sucking, and breathing cycles. In high-risk neonates, this coordination is rarely coordinated well before 34 weeks of gestation (5). Neonates who have difficulty achieving independent nutrition will usually experience low weight gain and may be exposed to malnutrition, delayed hospital discharge, and long-term health problems (6).
2. Objectives
Given the importance of complete oral feeding in preterm neonates, as well as the use of antibiotics in the first days of premature infancy and its effect on the gastrointestinal tract, this study investigated the correlation between antibiotic therapy and the timing of complete oral feeding in premature neonates admitted to the Neonatal Intensive Care Unit (NICU).
3. Methods
In this retrospective descriptive study, the study population included all premature neonates admitted to the NICU of Mahdieh Hospital, affiliated with the Shahid Beheshti University of Medical Sciences. After obtaining permission from the Ethics Committee (IR.SBMU.RETECH.REC.1399.319), the researcher referred to Mahdieh Hospital and received the file of all neonates admitted to the two NICUs in the form of an Excel file. Using the electronic system available in the hospital, the researcher had access to the files of neonates admitted to two NICUs from the beginning of October 2019 to the beginning of October 2020. A researcher-made questionnaire that included neonate’s demographic information, including gestational age, gender, type of delivery, Apgar score, date of birth and discharge, birth weight, and neonatal weight gain process, was prepared. It also collected information about the neonate’s treatment process, including types and duration of antibiotic therapy, blood culture date and result, type of ventilation (invasive or non-invasive), neonatal resuscitation, and other medical problems, such as jaundice, pneumothorax, retinopathy of prematurity (ROP), which may increase the duration of hospitalization, date of onset of oral feeding, date of complete oral feeding, etc. The type of neonate feeding, the duration of total parenteral nutrition (TPN), and gavage feeding were also recorded. Moreover, information about underlying diseases and medications taken by the mother during pregnancy and delivery, including gestational or pre-pregnancy diabetes, hypertension, preeclampsia, thyroid hormone disorder, urinary tract infection (UTI), premature rupture of membrane (PROM), and the type of antibiotics and steroid drugs during pregnancy was also included in the questionnaire.
Inclusion criteria were as follows: Gestational age between 30 and 37 weeks, hospitalization in the NICU, starting oral feeding during hospitalization, neonates who were ordered to be fed orally and by gavage simultaneously, and neonates with necrotizing enterocolitis (NEC) (to investigate the association between antibiotic therapy and the incidence of the disease). Also, exclusion criteria included premature neonates with incomplete patient records and those transferring from other centers because of a lack of sufficient background information, premature neonates with anomalies of the mouth and gastrointestinal tract, and those who had been fed orally for the first time. Furthermore, neonates who had complete cardiopulmonary resuscitation at birth or during treatment, premature neonates who died, those who were discharged with personal consent, and premature neonates who had been referred to other hospitals for further treatment were also excluded.
During data collection, information about the type of antibiotic and the duration of their use was obtained from the physician's orders and finally checked with the information in the medication card. This procedure was also performed to obtain the start date of oral feeding and complete oral feeding. Weight gain was also obtained from the files on the day of starting oral feeding, complete oral feeding, and the day of discharge. Also, in this research, the operational definition of tolerance of complete oral feeding was considered to be at least 100 to 120 mL/kg/day (7).
Data were analyzed using SPSS26. Univariate tests (One-way ANOVA and chi-square) were used to evaluate the correlation between the duration of antibiotic therapy and neonatal or maternal variables. A P < 0.05 was considered statistically significant.
4. Results
Out of the 712 reviewed patient records, information of 340 cases was assessed. Out of 340 studied patients, 55.6 were male, and 44.4% were female. For 2.9% of neonates, antibiotics were not prescribed. Moreover, 62.1% and 35% of neonates received antibiotics for less than five days and five and more days, respectively. Ampicillin was the most frequent antibiotic prescribed for patients at the rate of 63.7%. In addition, 79.8% of neonates had icterus, and the duration of hospitalization was 21.28 ± 19.08 days; hospital stay in 34.8% of patients was more than 20 days. Other descriptive variables are presented in Table 1.
| Variables | Values |
|---|---|
| Gender | |
| Male | 178 (55.6) |
| Female | 142 (44.4) |
| Duration of antibiotic therapy | |
| Never | 10 (2.9) |
| Less than five days | 211 (62.1) |
| Five days and more | 119 (35) |
| Type of antibiotics | |
| Ampicillin | 212 (63.7) |
| Cefotaxime | 81 (24.3) |
| Amikacin | 28 (8.4) |
| Gentamicin | 9 (2.7) |
| Vancomycin | 2 (0.6) |
| Meropenem | 1 (0.3) |
| Icterus | |
| Yes | 264 (79.8) |
| No | 67 (20.2) |
| Duration of hospitalization | 21.28 ± 19.08 |
| Less than three days | 2 (0.6) |
| 3 – 7 days | 42 (12.7) |
| 7 – 10 days | 80 (24.2) |
| 10 – 20 days | 91 (27.6) |
| 20 days and more | 115 (34.8) |
| Duration of total parenteral nutrition | 6.17 ± 6.36 |
| Duration of enteral feeding | 7.91 ± 11.14 |
| Duration of oral feeding | 8.27 ± 7.92 |
Descriptive Characteristics of Neonatal Variables a
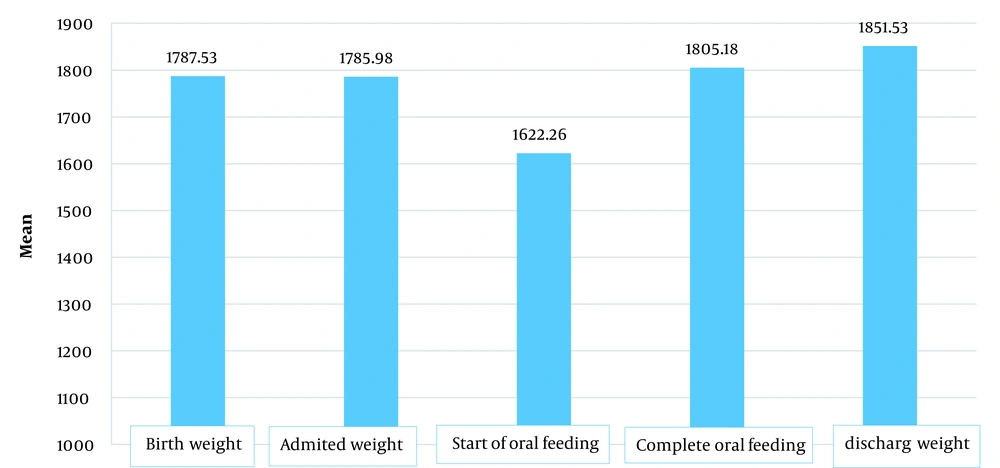
As shown in Figure 1, the average birth weight of neonates was 1787 g, and the weight on admission was 1785 g. The neonates had the lowest weight at the time of starting oral feeding, followed by at the time of complete oral feeding, and neonatal weight increased at the discharge time.
Table 2 provides the correlation between the duration of antibiotic therapy and feeding. Increasing the duration of antibiotic therapy led to a significant decrease in tolerating complete oral feeding (P < 0.05). Moreover, the duration of oral feeding was different according to the type of antibiotics. The duration of oral feeding in the neonate who received cefotaxime, amikacin, and vancomycin was more than in other antibiotic types (ampicillin, gentamicin, and meropenem) (P < 0.05).
As shown in Table 3, the one-minute Apgar score was higher in neonates who had been taking antibiotics for five days and more (P = 0.047). According to Table 4, there was no relationship between other neonatal qualitative variables (such as neonatal sex, mother's disorders, and antibiotics used by mothers) and the duration of antibiotic therapy (P > 0.05).
| Variables | Duration of Oral Feeding | Tolerance of Oral Feeding Weight |
|---|---|---|
| Duration of antibiotic therapy | ||
| Never | 7.25 ±5.96 | 1970 ± 311.127 |
| Less than five days | 8.27 ± 8.79 | 1850.83 ± 380.73 |
| Five days and more | 8.29 ± 6.23 | 1723.44 ± 307.14 |
| F-test | 0.03 | 3.66 |
| P-value | 0.967 | 0.027 |
| Type of antibiotics | ||
| Ampicillin | 6.62 ± 6.01 | 1823.61 ± 352.69 |
| Cefotaxime | 11.78 ± 10.12 | 1708 ± 290.03 |
| Amikacin | 11.74 ± 5.79 | 1696.75 ± 372.89 |
| Gentamicin | 9.50 ± 6.36 | 1857.50 ± 166.17 |
| Vancomycin | 11.22 ± 10.82 | 1795.63 ± 380.32 |
| Meropenem | 10.00 | 2020 |
| F-test | 5.62 | 0.59 |
| P-value | < 0.001 | 0.705 |
Comparison Between Duration of Antibiotic Therapy and Feeding Variable a
| Variables | Never | Less than Five Days | Five Days and More | F-test | P-Value |
|---|---|---|---|---|---|
| Gestational age | 32 ± 4.69 | 32.05 ± 2.71 | 31.81 ± 2.26 | 0.35 | 0.708 |
| Tolerance of feeding weight | 1685 ± 122.2 | 1643.24 ± 376.99 | 1589.03 ± 363.82 | 0.48 | 0.621 |
| Apgar 1 | 6.25 ± 2.75 | 7.84 ± 1.43 | 7.63 ± 1.45 | 2.93 | 0.047 |
| Apgar 5 | 8.25 ± 1.71 | 9.12 ± 1.33 | 9.04 ± 1.07 | 1.05 | 0.351 |
| IV line | 10 ± 8.48 | 12.09 ± 8.56 | 12.22 ± 7.99 | 0.07 | 0.928 |
| Umbilical venous catheter (UVC) | 6 ± 1.3 | 8.86 ± 6.04 | 12.67 ± 11.06 | 0.39 | 0.690 |
| Duration of total parenteral nutrition | 6.00 ± 2.83 | 6.53 ± 6.97 | 5.60 ± 5.31 | 0.65 | 0.523 |
| Duration of enteral feeding | 19.67 ± 22.05 | 7.81 ± 11.51 | 7.78 ± 10.06 | 1.69 | 0.185 |
Effect of Neonatal Quantitative Variables on the Duration of Antibiotic Therapy a
| Variables | Never | Less than Five Days | five Days and More | Chi-Square | P-Value |
|---|---|---|---|---|---|
| Gender | 0.97 | 0.326 | |||
| Male | 3 (1.7) | 113 (63.5) | 62 (34.8) | ||
| Female | 1 (0.7) | 85 (59.9) | 56 (39.4) | ||
| CPR | 0.17 | 0.919 | |||
| Yes | 0 | 4 (66.7) | 2 (33.3) | ||
| No | 4 (1.3) | 199 (63.4) | 111 (35.4) | ||
| Mother’s disorder | 1.45 | 0.227 | |||
| Yes | 3 (1.7) | 106 (59.6) | 69 (38.8) | ||
| No | 1 (0.7) | 97 (68.3) | 44 (31.0) | ||
| Maternal antibiotics | 1.62 | 0.202 | |||
| Yes | 0 | 88 (61.5) | 55 (38.5) | ||
| No | 4 (2.2) | 117 (64.3) | 61 (33.5) |
Effect of Neonatal Qualitative Variables on the Duration of Antibiotic Therapy a
5. Discussion
This study determined the correlation between antibiotic therapy and the complete oral feeding tolerance in premature neonates admitted to the NICU. The timing of antibiotic therapy in neonates was divided into three groups: The first group of neonates who did not take antibiotics, the second group of fewer than five days, and the third group of more than five days of antibiotics. There was a significant reverse correlation between the duration of antibiotic therapy and neonatal weight when tolerating oral feeding. This means that neonates who took antibiotics for more than five days weighed less when tolerating oral feeding than neonates in the other two groups. However, there was no significant correlation between the duration of antibiotic therapy and the duration of oral feeding. No study has yet examined the correlation between the duration of antibiotic therapy and weight while tolerating oral feeding, but in related studies, the correlation between antibiotic therapy and other neonatal outcomes has been examined. Martinez et al. (7) examined the effect of early exposure to antibiotics on feeding tolerance in preterm neonates and showed that neonates in the intervention group had a longer TPN feeding time than the controls. In this study, neonatal data that met the inclusion criteria were collected using the same nutrition guideline from five different centers. Also, 834 neonates were included in the control group, and 67 neonates were included in the intervention group. Besides, 10 to 20 cc of neonate feeding per day was increased regardless of weight, and when the neonate could tolerate 100 to 120 cc of intestinal feeding, complete intravenous feeding was discontinued. The timing of complete cessation of intravenous feeding was used as a comparison criterion for neonate feeding tolerance. The results showed that the neonates in the intervention group had a longer feeding time with TPN compared to the control group, which led to a delay in the onset of oral feeding and subsequent tolerance of oral feeding in the neonate. Generally, early antibiotic therapy has a negative effect on the neonate's feeding tolerance and can delay it, resulting in the premature neonate's weight gain. In this respect, Martinez's study is consistent with the present study. However, in this study, the antibiotic type and the exposure duration were not mentioned; an important point was considered in the present study (7). In a retrospective study conducted by Fajardo et al., premature neonates who received antibiotics more than five days after birth were underweight, thinner, and slower to grow. These findings are consistent with the results of the present study, as the use of antibiotics for more than five days in a newborn can be associated with low birth weight (8). However, more studies are required to prove the exact correlation between oral feeding and neonate weight in order to prove this.
On the other hand, in a retrospective study conducted by Reid et al. to investigate the correlation between early exposure to antibiotics and the growth rate of preterm neonates, no significant correlation was found between neonate weight and duration of antibiotic therapy (9). In the present study, 438 neonates who were eligible for the study were divided into three groups: The first group consisted of 58 neonates who did not receive any antibiotics, the second group entailed 304 neonates receiving antibiotic therapy for less than five days, and the third group contained 69 neonates receiving more than five days of antibiotic therapy. The difference in birth weight and discharge day was very significant in group two, but the Z-score of weight among the three groups was not statistically significant. Both findings of the study above were inconsistent with the results of the present study. One of the reasons for this discrepancy may be attributed to the difference in fetal age of studied neonates. In the present study, neonates over 25 weeks were included, but in the mentioned study, neonates aged 30 to 32 weeks were studied. The reason for the insignificance of neonatal weight in this study can be attributed to the age difference between neonates, and possibly the neonates with less fetal age will have a more noticeable weight difference.
Several studies have assessed the correlation between antibiotic therapy and other neonatal outcomes, in which complications, such as bronchopulmonary dysplasia (BPD), ROP, etc., have been examined. For example, Flannery et al. investigated the early exposure of neonates to antibiotics and its association with BPD and neonate mortality. They revealed no significant correlation between the duration of antibiotic therapy and BPD and neonatal mortality. In this study, out of 4950 neonates, 3946 cases received antibiotics in the first week after birth, and 1004 neonates did not receive antibiotics (10). Moreover, Greenberg et al. did not report any significant association between long-term antibiotic use and the incidence of NEC and neonatal death (11). However, Ting et al. investigated the consequences of antibiotic therapy in very low birth weight neonates and showed that the longer the duration of antibiotic exposure in underweight neonates, the higher the mortality rate and the incidence of BPD, chronic lung disease (CLD), and ROP (12). Furthermore, Cantey et al. evaluated antibiotic therapy for preterm neonates with very low birth weight and its consequences. They suggested that, no matter how long the premature neonate is exposed to antibiotics after treatment, the longer the acute period of the disease, the higher the risk of sepsis, NEC, and death (13). It can be concluded that antibiotic therapy may be effective for malnourished and low birth weight neonates and outcomes, such as NEC, BPD, and death; yet, there is no clear association between neonates with better physiological status. More detailed studies with appropriate sample sizes are needed to determine this correlation.
We found a significant correlation between the type of antibiotic used for the neonate and the duration of his/her oral feeding. The duration of oral feeding was longer in neonates taking cefotaxime, amikacin, and vancomycin than other antibiotics. However, there was no significant correlation between the antibiotic type and the weight gain process in the neonate while tolerating oral feeding. In this regard, Armanian et al. investigated the effect of a moderate dose of erythromycin on the treatment of malnutrition in preterm neonates. They showed that erythromycin at a moderate dose (20 mg/kg/day) was effective in reducing the frequency of intolerance to oral feeding and improvement of the mean time to reach full milk volume in low birth weight preterm neonates (14). The mentioned study is consistent with our study reporting that the type of antibiotic can affect the neonate's feeding tolerance and its duration, as some antibiotics of a certain group can even increase the feeding tolerance in premature neonates and improve their condition. In the study by Cairns et al., neonates treated with low-dose intravenous erythromycin had a shorter time to receive intestinal nutrition than those treated with the placebo (15). In another study by Nuntnarumit et al., the high oral dose of erythromycin in neonates less than 32 weeks of age was more effective in treating milk intolerance in the case group than in the placebo group (16).
5.1. Conclusions
Generally, the results showed that the type of antibiotic could affect the tolerance time of neonate oral feeding regardless of the duration of antibiotic therapy. Besides, the length of time the neonate is treated with antibiotics is critical because prolonging the treatment and exposure to antibiotics after the acute period of the disease can cause the neonate to lose more weight and be at greater risk for infections, illnesses, and even death.