1. Background
Kawasaki disease (KD) is a common pediatric disease that involves systemic medium- and small-vessel vasculitides, manifested as acute fever and rash. It mainly occurs in children younger than five years old, with various severities (1). About 15% of KD children are complicated with coronary artery injury, and their growth is adversely affected if the treatment opportunity is missed. Moreover, coronary artery injury eventually progresses into acquired heart disease, seriously threatening children’s quality of life. Severe KD cases are often accompanied by acute heart failure or acute coronary syndrome, which eventually leads to death (2, 3). At present, intravenous immunoglobulin (IVIG) remains the first choice for the clinical treatment of KD, but not all children respond to IVIG. Approximately 10-15% of KD children respond to IVIG (4), also known as "IVIG-unresponsive KD", who are more prone to coronary artery injury (5).
2. Objectives
Considering that the risk factors for non-response to the initial dose of IVIG and coronary artery injury may differ between Chinese and foreign populations due to genetic background, living environment, and medical level differences, it is necessary to explore suitable prediction methods for Chinese children. Thereby motivated, the clinical data of 222 KD children treated with IVIG at the initial dose of 1 g/(kg·d) twice or 2 g/(kg·d) once in our hospital from January 2016 to January 2021 were retrospectively studied. The clinical characteristics of KD children without response to the initial dose of IVIG were analyzed, and the predictors for non-response were explored, aiming to provide valuable evidence for clinical treatment.
3. Methods
3.1. General Data
A total of 222 children with KD were enrolled as subjects, including 142 boys and 80 girls aged 4 months to 7 years old, with a hospital stay length of 8 - 22 d. They were diagnosed as KD in accordance with the criteria formulated by the American College of Cardiology and the American Academy of Pediatrics (6). According to the recommendations of the National Symposium on KD in China (2007), a child who has fever for 5 days or longer with at least two clinical manifestations of KD and significantly increased inflammatory indicators and without other diseases can be diagnosed as incomplete KD. Following the diagnostic criteria recommended by National Symposium on KD in China (2007), IVIG-unresponsive KD is defined as follows (7): except for secondary infections, fever lasts for 36 h after IVIG therapy (body temperature ≥ 38°C) or symptoms (fever and at least one KD symptom) reappear within 2 - 7 d after medication. Exclusion criteria: patients who cannot be definitely determined as IVIG non-responders.
3.2. Grouping Criteria
After diagnosis, the children were administered with IVIG (2 g/kg; Jiangxi Boya Bio-pharmaceutical Group Co., Ltd., China) at the initial dose of 1 g/(kg·d) twice or 2 g/(kg·d) once. According to the changes in body temperature, they were divided into sensitivity and non-response groups. In the sensitivity group, body temperature returned to normal within 36 h after medication, and white blood cell count (WBC) and C-reactive protein (CRP) levels were gradually reduced about one week later, without recurrence during the disease course. In the non-response group, except for the secondary infections, fever lasted for 36 h after IVIG therapy (body temperature ≥ 38°C), or symptoms reappeared within 2 - 7 d after medication.
3.3. Observation Indicators
The medical records of KD children were referred, and their gender, age, main clinical manifestations, and disease history were collected. Defervescence after the initial dose of IVIG was regarded as an indicator for treatment outcome. Fasting venous blood was extracted from each child within 24 h after admission, followed by laboratory examinations, including (A) blood routine test: WBC, percentage of neutrophils (No. (%)), hemoglobin (Hb) and platelets (PLT); (B) CRP and erythrocyte sedimentation rate (ESR); (C) blood biochemical test: alanine aminotransferase (ALT), lactate dehydrogenase (LDH), serum albumin (ALB), total bilirubin (TB) and blood sodium (Na+). Whether coronary artery disease occurred was observed according to echocardiographic findings.
3.4. Statistical Analysis
Data analysis was administered using SPSS 26.0. All measurement data were subjected to the homogeneity of variance and normal distribution tests. The measurement data with normal distribution were expressed as mean ± standard deviation (
4. Results
4.1. General Data and Clinical Characteristics
Of 222 included children, 181 (81.53%) were in the sensitivity group, and 41 (18.47%) were in the non-response group. There was no statistically significant difference between the study groups concerning variables of gender, age, body mass index (BMI), and main clinical manifestations (P > 0.05). The main clinical manifestations of the non-response group were fever, lip changes, conjunctival hyperemia, extremity changes, erythema multiforme, and enlargement of cervical lymph nodes in a descending order. The non-response group had a significantly higher proportion of patients with a body temperature of > 40°C in comparison to the sensitivity group (P < 0.05). Based on the echocardiographic images, the incidence rate of coronary artery disease in the non-response group was significantly higher than that of the sensitivity group (P < 0.05) (Table 1).
| Indicator | Sensitivity Group (N = 181) | Non-response Group (N = 41) | χ2/t/Z | P-Value |
|---|---|---|---|---|
| Sex (Male) | 108 (59.67) | 25 (58.14) | 0.034 | 0.854 |
| Age (mo) | 27.89 ± 4.56 | 28.71 ± 4.62 | t = 1.021 | 0.308 |
| Age < 12 mo | 20 (11.05) | 9 (20.93) | χ2 = 3.010 | 0.083 |
| BMI (kg) | 13.26 ± 1.92 | 13.45 ± 1.11 | t = 1.168 | 0.244 |
| Duration of fever before first IVIG (d) | 5 (5~6) | 6 (4~7) | Z = 0.784 | 0.352 |
| Duration of fever ≥ 5 d | 170 (93.92) | 43 (100.00) | χ2 = 2.748 | 0.094 |
| Body temperature > 40°C | 55 (30.39) | 26 (60.47) | χ2 = 4.056 | 0.044 |
| Length of hospital stay (d) | 13.00 ± 2.34 | 16.71 ± 2.52 | t = 9.207 | < 0.001 |
| Incomplete KD | 27 (14.92) | 8 (18.60) | χ2 = 0.358 | 0.549 |
| Conjunctival hyperemia | 164 (90.61) | 37 (86.05) | χ2 = 0.785 | 0.376 |
| Lip changes | 165 (91.16) | 38 (88.37) | χ2 = 0.318 | 0.573 |
| Erythema multiforme | 127 (70.17) | 33 (76.74) | χ2 = 0.737 | 0.391 |
| Enlargement of cervical lymph nodes | 121 (66.85) | 27 (62.79) | χ2 = 0.256 | 0.613 |
| Extremity changes | 150 (83.43) | 35 (81.40) | χ2 = 0.053 | 0.818 |
| Coronary artery disease | 42 (23.20) | 20 (46.51) | χ2 = 9.429 | 0.002 |
General Data and Clinical Characteristics of KD Children in Sensitivity and Non-response Groups Receiving IVIG Therapy a
4.2. Two Different Drug Administration Methods of IVIG
Of 222 children, 80 were administered with IVIG at a dose of 1 g/(kg·d) twice, among whom there were 24 cases (30.00%) with non-response to IVIG and 22 cases (27.50%) with coronary artery disease. The remaining 142 cases were treated with IVIG at a dose of 2 g/(kg·d) once, among whom there were 19 cases (13.38%) with non-response to IVIG and 40 cases (28.17%) with coronary artery disease. The probability of non-response in children treated at a dose of 2 g/(kg·d) once was significantly lower than that of children treated at a dose of 1 g/(kg·d) twice (P < 0.05), but the incidence rate of coronary artery disease was similar among them (P > 0.05).
4.3. Laboratory Test Results
No. (%), PLT count, and CRP level were higher and ALB level was significantly lower in the non-response group than that of the sensitivity group (P < 0.05) (Table 2).
| Indicator | Sensitivity Group (N = 181) | Non-response Group (N = 41) | t | P-Value |
|---|---|---|---|---|
| WBC (×109/L) | 19.65 ± 1.67 | 20.16 ± 1.59 | 1.816 | 0.070 |
| N proportion | 0.73 ± 0.06 | 0.75 ± 0.05 | 2.024 | 0.044 |
| Hb (g/L) | 107.35 ± 5.46 | 107.88 ± 5.52 | 0.571 | 0.569 |
| PLT (×109/L) | 353.41 ± 10.24 | 374.18 ± 11.25 | 11.729 | < 0.001 |
| ESR (mm/1h) | 71.88 ± 2.46 | 72.11 ± 2.54 | 0.548 | 0.584 |
| CRP (mg/L) | 74.39 ± 2.68 | 92.31 ± 2.74 | 39.246 | < 0.001 |
| LDH(IU/L) | 331.27 ± 26.19 | 335.26 ± 2.28 | 0.996 | 0.330 |
| ALB (g/L) | 34.22 ± 3.28 | 31.78 ± 2.04 | 4.664 | < 0.001 |
| TB (IU/L) | 31.25 ± 3.22 | 31.46 ± 2.87 | 0.392 | 0.695 |
| ALT (IU/L) | 60.64 ± 4.52 | 61.13 ± 4.32 | 0.644 | 0.520 |
| Na+ (mmol/L) | 134.22 ± 18.27 | 132.46 ± 19.22 | 0.562 | 0.575 |
Main Laboratory Test Results of KD Children in Sensitivity and Non-response Groups Receiving IVIG Therapy
4.4. Logistic Regression Analysis Results of Related Factors for IVIG-unresponsive KD
Multivariate logistic regression analysis results revealed that increased No. (%) and CRP level, as well as reduced ALB level, were independent risk factors for the non-response to the initial dose of IVIG (P < 0.05) (Table 3).
| Factor | β | SE | OR | 95% CI | P-Value |
|---|---|---|---|---|---|
| No. (%) | 0.157 | 0.064 | 1.132 | 1.022~1.356 | 0.012 |
| PLT | 0.012 | 0.006 | 1.011 | 0.994~1.032 | 0.134 |
| CRP | 0.104 | 0.021 | 1.094 | 1.049~1.178 | < 0.001 |
| ALB | -0.232 | 0.115 | 0.658 | 0.614~0.984 | 0.032 |
Logistic Regression Analysis Results of Related Factors for IVIG-unresponsive KD
4.5. Cut-off Values of Independent Factors for IVIG-unresponsive KD
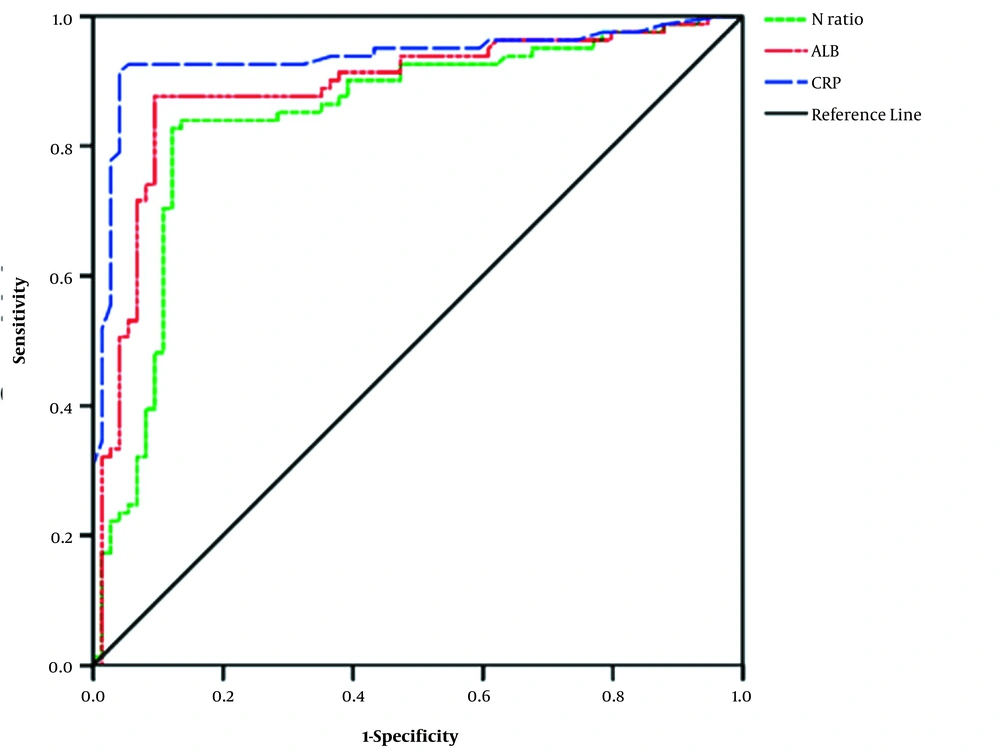
ROC curve analysis results showed that the AUC values of No. (%), CRP, and ALB were 0.734, 0.867, and 0.784, respectively, indicating high diagnostic values. If the cut-off value of No. (%) was 0.725, the sensitivity and 1-specificity were 0.911 and 0.457, respectively (P < 0.001). If the cut-off value of CRP was 78.43 mg/L, the sensitivity and 1-specificity were 0.848 and 0.324, respectively (P < 0.001). If the cut-off value of ALB was 32.89 g/L, the sensitivity and 1-specificity were 0.784 and 0.405, respectively (P < 0.00). Therefore, physicians should be wary of possible non-response to the initial dose of IVIG in KD children with No. (%) ≥ 0.725, CRP ≥ 78.43 mg/L or ALB ≤ 32.89 g/L (Figure 1).
4.6. Treatment of KD Children in Non-response Group
In the non-response group (n = 41), IVIG at 2 g/(kg·d) was administered 36 - 72 h later again. Then, the body temperature of 33 cases was controlled, while it still fluctuated in the other 9 cases and returned to normal after treatment with glucocorticoids.
5. Discussion
At present, the etiology of KD remains unclear, but epidemiological characteristics indicate that infection and immune dysfunction may be important mechanisms (7). Abnormal immune response-mediated vasculitis is considered an important pathogenesis of KD, and the aim of IVIG therapy is to inhibit the abnormal activation of the immune system and inflammatory responses. Hence, in this study, the clinical manifestations of KD children with non-response and sensitivity to IVIG therapy were first compared to observe whether the range and degree of early vasculitis in different severities were related to the efficacy of IVIG. Except for the fever, among the five clinical characteristics in the non-response group, lip changes occurred most frequently, and enlargement of cervical lymph nodes was rarely observed, indicating that the degree of systemic manifestations of early vasculitis was not associated with IVIG efficacy, being consistent with the findings of Yang et al. (8). High-dose IVIG combined with aspirin, as the standard treatment regimen for KD, has been demonstrated to effectively relieve the acute symptoms of KD and to reduce the incidence rate of coronary artery disease. This study suggested that different drug administration methods were related to IVIG non-response. Compared with the children treated with IVIG at a dose of 1 g/(kg·d) twice, the incidence rate of non-response in children treated with IVIG at a dose of 2 g/(kg·d) once was significantly lower, being consistent with the results reported by Hamada et al. (9). In this study, the incidence rate of IVIG non-response was slightly higher (18.47%). Possibly, some children received IVIG at 1 g/(kg·d) twice, incomplete KD cases were not excluded, and the sample size was small. Uehara et al. (10) reported that the difference between the incidence rates of IVIG-unresponsive KD in various regions might also be related to genetic factors.
Unresponsive KD is typified by persistent fever and severe coronary artery disease, of which the former means persistent vascular inflammation and defects in suppressing immune stimulation (11). Considering that coronary artery disease can cause serious consequences, it is essential to strengthening the early management of unresponsive KD. A previous study confirmed that coronary artery disease incidence rate was higher in children with unresponsive KD (12). In this study, there were 20 cases (46.51%) with coronary artery disease in the non-response group, and the incidence rate was significantly higher than that of the sensitivity group (42 cases, 23.20%) (P < 0.05), indicating that IVIG-unresponsive KD cases were prone to coronary artery disease and the need for additional doses of IVIG and/or other therapies (4). Besides, the sequelae of KD may be related to subclinical atherosclerosis in adolescence (13). Therefore, recognizing and early treatment of these children are of great clinical significance.
The risk factors for IVIG-unresponsive KD are also associated with severe immune response after antigen stimulation (14, 15). Nakamura et al. reported that serum sodium might be the most useful predictor for KD-induced giant coronary aneurysms (16). Using multivariate logistic regression analysis, Egami et al. found that age, disease duration, PLT, ALT, and CRP were key predictors for IVIG resistance (17). Additionally, Kobayashi et al. reported that disease duration, age, No. (%), PLT, serum aspartate aminotransferase, sodium, and CRP significantly affected IVIG resistance (18). Furthermore, Tremoulet et al. found that higher percent bands, as well as levels of CRP, ALT, and gamma-glutamyl transferase were important factors affecting IVIG resistance (19). The results of multivariate logistic regression analysis herein indicated that increased No. (%) and CRP level, as well as reduced ALB level, were independent risk factors for non-response to the initial dose of IVIG.
An increase in neutrophils and a decrease in lymphocytes indicate the early tissue infiltration of effector T and B lymphocytes, which may be attributed to the genetic defect of activated immune cells (20). CRP is a cellular acute-phase reactant that increases in the acute phase and decreases in the recovery phase, displaying dynamic changes in the course of KD. Elevation in its level reflects the degree of inflammation. Lee et al. (21) reported that 11 (out of 91) KD children (12%) in South Korea had no response to the initial dose of IVIG, and the changes in CRP levels were valuable for predicting non-response. Sato et al. (22) reported that No. (%), IL-6, and CRP levels were significantly increased in the blood of KD children who had no response to IVIG. In this study, No. (%) and CRP significantly increased in KD children in the acute phase, especially in those with coronary artery dilation, suggesting that these two indicators were related to the acute inflammatory response and coronary artery dilation. On the other hand, low ALB level was associated with IVIG non-response, being in agreement with previous literature (23). In addition, low serum ALB level (≤ 29 g/L) has been reported to be an independent risk factor for IVIG-unresponsive KD (24). The vascular endothelial growth factor can directly act on vascular endothelial cells to induce their proliferation and increase vascular permeability (25); thus, leading to hypoalbuminemia and non-cardiogenic pulmonary edema. Hence, a low ALB level can predict the degree of vascular inflammation and exudation in children with acute KD. In this study, ROC curve analysis confirmed that physicians should be wary of possible non-response to the initial dose of IVIG in KD children with No. (%) ≥ 0.725, CRP ≥ 78.43 mg/L, or ALB ≤ 32.89 g/L. However, this was a single-center retrospective study with small sample size, and there were limitations in the cut-off value of each independent risk factor obtained from the ROC curve in predicting the efficacy of IVIG. Therefore, it is recommended to perform multicenter studies with larger sample sizes to construct predictors for IVIG efficacy on KD.
In summary, pediatricians should pay attention to possible non-response to the initial dose of IVIG in KD children with N ≥ 0.725, CRP ≥ 78.43 mg/L, or ALB ≤ 32.89 g/L.