1. Introduction
Biotin is a water-soluble molecule and a cofactor for four carboxylases involved in key steps of energy metabolism and fatty acid synthesis (1). BTD is an enzyme recycling the vitamin biotin, and the deficiency in this enzyme leads to subsequent neurologic manifestations that are occasionally irreversible (2).
The BTD deficiency (OMIM: 253260) with a wide phenotypic variation (3) is a treatable metabolic disorder with autosomal recessive inheritance, characterized by severe neurological and dermatological manifestations, if untreated (4). The BTD gene is found on chromosome 3p25.1 and consists of four exons totaling 9964 base pairs in length (5). It encodes a 523 amino acid glycosylated protein (UniProt ID: P43251) (uniprot.org/uniprot/P43251). Missense, nonsense, single and multiple nucleotide deletions, single and multiple nucleotide insertions, deletion/insertions, cryptic splice site mutations, and compound allelic mutations have all been reported in BTD deficiency patients (6). The prevalence of the BTD deficiency is 1 in 60000 births (7). However, we thought its prevalence is higher in Iran because of the increased rate of consanguineous marriage. If the pathogenic variations in the family are known, the alternatives can be carrier testing for at-risk family members and prenatal testing for pregnancies at elevated risk (3).
Profound BTD deficiency can mimic acquired demyelinating syndromes such as neuromyelitis optica spectrum disorder (NMOSD). This manuscript reports the first Iranian girl with profound BTD deficiency, flaccid quaderiplegia, and optic atrophy, who was misdiagnosed with NMOSD.
2. Case Presentation
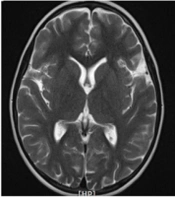
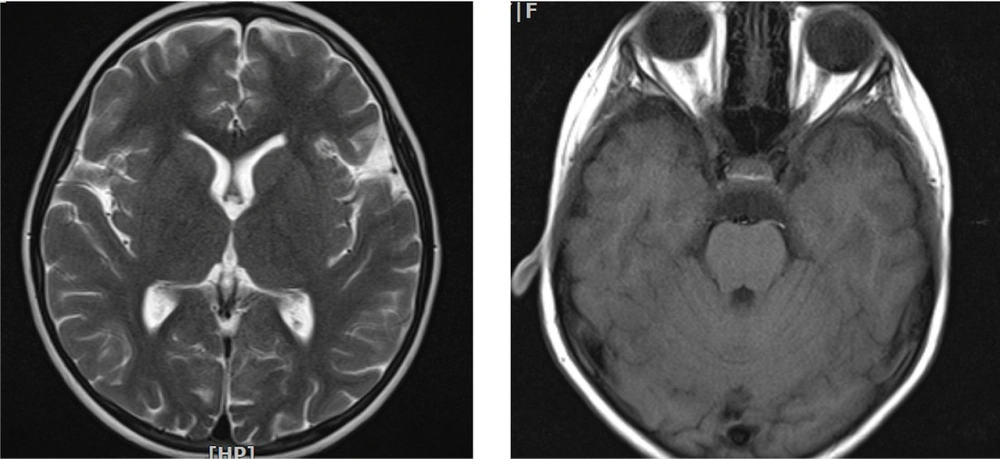
A six-year-and-ten-month-old girl referred to our clinic with complaints of gradual visual loss and progressive limb weakness represented in the past eight months ago. Her parents were first-degree cousins, and she had two healthy siblings. Developmental history was normal, except for frequent falling since four years ago. There was no history of hearing loss, cognitive dysfunction, or personality changes. She had two hospital admissions before, because of a decrease in visual acuity and progressive limb weakness, with normal brain MRI. She had also received immunosuppressive treatment in each admission and had been discharged without complete recovery. Finally, she referred to our tertiary center with the deterioration of weakness and painless gradual visual impairment, for complete evaluation and probably plasma exchange without definite diagnosis.
On her exam, she was bedridden, awareness was preserved, she had flaccid quaderiplegia, deep tendon reflexes in all four limbs were brisk, and bilateral optic atrophy in fundoscopy was detected. Other exams, including skin and hair evaluations, were normal.
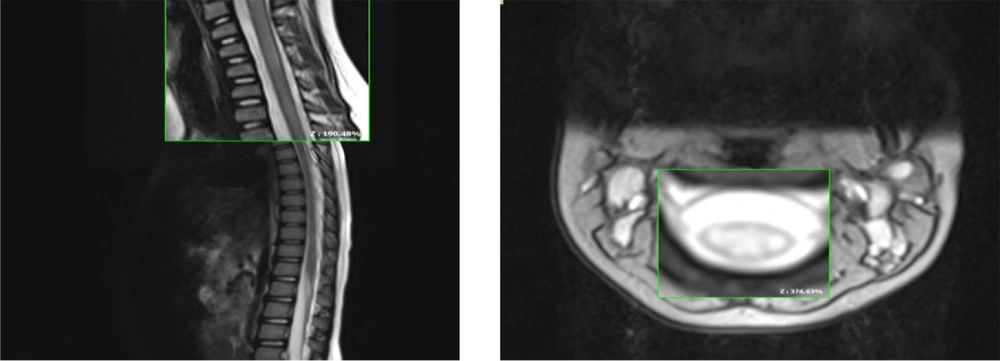
Spinal MRI revealed a longitudinally T2/FLAIR hyperintense signal change in the cervical cord from C2-C5 (Figures 1 and 2).
In laboratory investigations, blood lactate was 25 mg/dL, and cerebrospinal fluid (CSF) lactate was 61 mg/dL. Other laboratory tests, such as the titer of serum MOG Ab and NMO Ab were negative. CSF IgG index was 1200 mg/dL; however, we could not investigate CSF MOG Ab.
Regarding the likelihood of seronegative neuromyelitis optica spectrum disorder (NMOSD) and because of poor response to treatment and disease progression, she underwent plasma exchange; However, after ten courses of plasma exchange, recovery was poor, and there was no improvement. Considering the CSF lactate elevation, we asked for other metabolic tests such as tandem mass spectrometry and urine organic acid profile. At follow-up, tandem mass spectrometry displayed a profound decrease in the BTD activity, confirmed by genetic study. Molecular genetic testing was run for the sample. Genomic DNA sequencing was extracted from the peripheral blood sample, and direct DNA sequencing was performed for four coding exons and 20 bases of flanking non-coding regions of the BTD gene (NM_001370658.1). The sequences of the primers are presented in (Table 1). A homozygous missense variant, c.838A>C (p.Asn280His) in the BTD gene, was detected in the results of the sanger sequencing. This variant has previously been classified as pathogenic in the Clinvar database (VarID: 156004). Moreover, the VarSome prediction tool and the multiple lines of the in-silico computational analysis (CADD, DANN, MutationTaster, PolyPhen, and others) support the pathogenic effect of this variant on the gene product. The allelic frequency of this variant in the GnomAD database is about 0.00000398. According to the ACMG guideline, this mutation was classified as the pathogenic category with PM1, PM2, PP2, PP3, and PP5 evidence (Table 2). The mutation of the BTD gene is associated with profound BTD deficiency with autosomal recessive inheritance.
| Gene | Primer Sequence (5' - 3') | PCR Product (bp) |
|---|---|---|
| BTD | GGTGGTCTCAATCTCCTGAC | 892 |
| GTGGAGATAGCCTTCCTTTC |
| Gene/Transcribe | Consanguinity | DNA Change | Type | Protein Change | dbSNP | Exon Number | Zygosity | Clinvar | Prediction Tools |
|---|---|---|---|---|---|---|---|---|---|
| BTD (NM_001370658.1) | FC | c.838A>C | missense | p.Asn280His | rs587783006 | Ex 4 | HOM | Path (ID: 156004) | Damaging |
Then we discontinued plasma exchange and began high dose oral biotin 20/mg/daily (four times a day). During the first two weeks, there was a marked improvement in motor function in response to oral biotin. Four weeks after treatment, she could walk 20 to 30 steps independently. Two months after rehabilitation, she restored full ambulation, and spinal MRI findings appeared normal. Visual acuity improved partially in both eyes after eight months, and after two years, follow-up remained unchanged.
3. Discussion
Biotin as a B-complex vitamin acts as a coenzyme in four carboxylation reactions (namely pyruvate carboxylase, propionyl-CoA carboxylase, and 3-methylcrotonyl CoA-carboxylase, and acetyl-CoA carboxylase) in humans, with the first three located in the mitochondrion and the last one in the cytosol. The aforementioned enzymes play a critical role in energy metabolism. Several proteolytic enzymes degrade the holocarboxylase to form biocytin in the lysosomes hydrolyzed by BTD to form biotin and lysine. BTD deficiency makes this process fail, resulting in reduced biotin uptake and impaired carboxylase function (7).
According to the results of the worldwide screening of BTD deficiency, the incidence of the disorder is 1 in 61,067 cases for the combined incidence of profound and partial BTD deficiency (8). In this regard, to the best of our knowledge, the prevalence of the BTD deficiency in Iran has not been studied yet; however, it is estimated to be high due to the increased rate of consanguineous marriages. The age of symptom-onset for BTD deficiency vary from infancy to adulthood. Seizures, hearing loss, skin rash, hypotonia, alopecia, ataxia, respiratory problems, optic atrophy, and developmental delay in untreated children with profound enzyme deficiency (serum BTD activity < 10% of normal) are some symptoms of the classical form of the disease, among these symptoms, hearing loss, optic atrophy, and developmental delay can be irreversible; however, a majority of these clinical findings improve with biotin replacement therapy.
Furthermore, late-onset BTD deficiency can present by different phenotype from the classical form; however, it may manifest with similar clinical symptoms, such as limb weakness and vision problems (4, 8). In recent years, there have been several case repots on this disorder with uncommon manifestations, including recurrent myelopathy and spinal cord involvement mimicking acquired demyelinating syndromes; however, they often do not respond to immunosuppressive treatment (9-12). Our patient developed myelopathy with optic neuropathy, misleading to the NMOSD diagnosis; however, despite two courses of methylprednisolone and ten courses of plasma exchange, she was not recovered. This unusual manifestation could often be noticed in the late-onset form and in countries with no neonatal screening programs.
Although NMOSD should be considered in all individuals by longitudinally extensive spinal cord involvement (T2 high signal in at least three vertebral segments), as our patient, but there are several points to distinguish between NMOSD and BTD deficiency. For example, the disease course in our patient was gradual; however, this is often relapsing-remitting in NMOSD. Moreover, the other differences are as follows: optic atrophy in our patient versus optic neuritis in NMOSD patients, the dominant pattern of brain imaging in NMOSD by desire with aquaporin receptors site in brain unlike BTD, and also no response to standard treatment in NMOSD.
On the other hand, in a recent trial of progressive multiple sclerosis (MS) patients with high-dose biotin, there was no clear improvement in clinical disease course (1). However, in any individual with acquired demyelinating syndromes such as NMOSD and MS who remarkably improve with high dose biotin, late-onset BTD deficiency should be evaluated (13, 14).
The mentioned mutation was reported in the BTD database in the ARUP Laboratory in Michigan, USA, from 1988 to the end of 2012. Above 3.25 million newborns were screened during this period, and 32 cases were identified with profound BTD deficiency, and 197 cases were identified with partial BTD deficiency (15). Among these 197 patients with the partial form, two siblings, had mutations similar to the variation in our case. Although our patient had profound BTD deficiency, the two reported cases had partial BTD deficiency. This difference may be due to phenotype-genotype correlation in our studied case. Our patient had a homozygous variant c.838A>C (p.Asn280His) with neurologic and ophthalmologic manifestations. In contrast, the two reported cases had a compound heterozygote for two variants, p.Asp424His and p.Asn280His in the BTD gene, with a partial phenotype. This missense variant is a semi-conservative amino acid substitution, which may affect secondary protein structure to produce a non-functional enzyme in the biotin cycle (http://evs.gs.washington.edu/EVS/). Furthermore, this variant has not been reported in the Iranome databases (http://www.iranome.ir), as an Iranian genomic variant database. Moreover, a severe form of the disease was reported in an Iranian consanguineous family with a novel homozygous pathogenic variation c.528_542del15 (p.Asn197_ Ser201del) in the BTD gene (16). In conclusion, we considered our patient the first case with this variant and the symptoms of profound BTD deficiency among the Iranian population.
Due to the high rate of consanguineous marriages in Iran (30 - 39%) (17) and the lack of a newborn screening program, undiagnosed patients are more prevalent; probably, late onset biotinidase deficiency with unusual clinical feature and neuroimaging, can mimic NMOSD, MS, and so on. Genetic counseling should be done when a patient is diagnosed with BTD deficiency. The measurement of BTD enzyme activity and molecular genetic tests should be considered to assess the relatives at risk (18). Accordingly, serum and CSF lactate should be checked in any patients suspected of acquired demyelinating syndromes, and BTD deficiency should be considered as well.
3.1. Conclusions
In summary, in any patient with longitudinally extensive cord involvement not responding well to the conventional treatment, BTD deficiency should be considered, and serum/CSF lactate should be measured.