1. Context
Cystic fibrosis (CF) is a lethal genetic disorder that affects 1 in 2500 newborns. An estimated 70,000 people live with CF worldwide (1). CF is caused by mutations in the CF transmembrane conductance regulator (CFTR) gene, eventuating in CFTR protein synthesis and function changes. A mutation in the ΔF508 CFTR protein is the most frequent CFTR mutant allele in CF patients. The CFTR protein is a cAMP-activated channel that regulates chloride and bicarbonate ion transport across certain epithelial cells. Thus, a dysfunctional CFTR protein causes abnormal salt and water transport in the form of thick and sticky secretions in several organs, especially the lungs, pancreas, liver, and gastrointestinal tract. Hence, CF patients manifest irreversible airway damage, exocrine pancreatic insufficiency (resulting in intestinal malabsorption), chronic infections (caused by trapping of bacteria in the airways), and intense inflammation (2). Persistent chronic inflammation finally leads to lung disease progression, the leading cause of death in these patients (3). Although treatments with various drugs improve the expression and function of the CFTR ion channel or increase the delivery of ΔF508-CFTR to the plasma membrane, data on the effect of these drugs on CF-related inflammation are limited (3-5). As a result, there has been an interest in using natural products with anti-inflammatory properties to treat CF patients, and many of those affected have taken up complementary and alternative medicine (6, 7). In this regard, curcumin possesses anti-inflammatory, antitumor, antioxidant, anti-atherosclerotic, and antimicrobial properties and is used as a complementary treatment for many diseases (8, 9). Recent studies have shown that curcumin influences a variety of ion channels and pumps (10, 11). However, in some studies, researchers have observed that curcumin has no significant effect on the functional correction of defective ΔF508-CFTR processing in transfected cells. Currently, one of the main challenges in the treatment of CF patients is activating the CFTR gene (especially the common mutant allele ΔF508). At the same time, reducing inflammation is another priority due to the role of inflammation in lung pathology and disease progression.
According to the mentioned properties of curcumin, and due to the lack of a systematic review study in this field, the purpose of this review was to summarize the effects of curcumin demonstrated in experimental models of CF. As there is only 1 clinical study on this topic (12), we focused on the experimental models.
2. Evidence Acquisition
2.1. Search Strategy
The present study reviewed the studies utilizing curcumin against CF published until July 2021. The electronic databases searched included PubMed, ScienceDirect, ProQuest, and Springer. The searches related to the primary research question were systematically performed. Descriptors were used to identify all articles that studied the effects of curcumin in experimental models of CF, including “curcumin,” “turmeric,” “cystic fibrosis transmembrane conductance regulator (CFTR),” and “cystic fibrosis.”
2.2. Inclusion and Exclusion Criteria
The studies selected for this review evaluated the effects of curcumin using in vitro CF disease models, including the effect of curcumin on CFTR expression or on the CFTR Cl- channel. Exclusion criteria were (i) articles not written in English, (ii) review articles, (iii) books, (iv) conference reports, (v) letters, and (vi) personal opinions.
2.3. Data Extraction
One author (F.F.R) searched and screened for eligible studies based on the inclusion and exclusion criteria. The data were extracted by 2 independent reviewers (F.F.R and E.F). This review presents the main characteristics of each study, such as the first author’s name, publication year, the experimental model, the dose of curcumin, and the outcome.
3. Results
3.1. Study Search and Selection
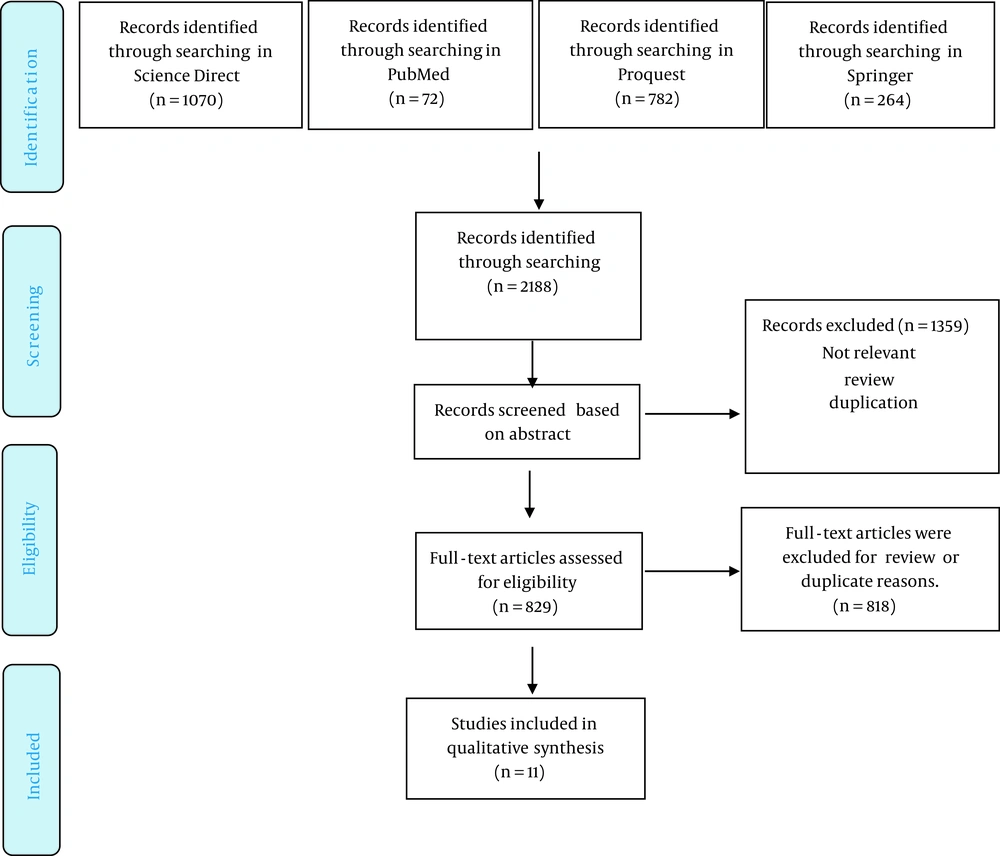
Based on our search of the literature, a total of 2188 studies were found (72 in PubMed, 1070 in ScienceDirect, 782 in ProQuest, and 264 in Springer). After evaluating the titles, we eliminated 1359 of these studies due to being irrelevant, duplicate entries, or review studies. Finally, after reading the abstracts, 11 articles were deemed eligible for this systematic review. The systematic procedures are presented in Figure 1.
3.2. Description of Studies
The articles studied in this review, shown in Table 1, were conducted in Japan (n = 2), Iowa City (n = 1), Sweden (n = 1), Birmingham (n = 1), France (n = 2), Germany (n = 1), New Haven (n = 1), Italy (n = 1), and the USA (n = 1). All articles were published in English from 2004 to 2019.
| Reference | Experimental Model | Interventions (Dosage and Duration of Treatment) | Main Findings |
|---|---|---|---|
| Chaudhary et al. (13) | Human bronchial epithelial CFBE41o | Curcumin (10, 20, 40, 60 μM; 1, 3, 6, 9, 12 h) | Curcumin suppresses mRNA, surface protein expression, and the function of TLR2 by accelerating SP1 degradation through an oxidative process |
| Bernard et al. (14) | HEK 293T/17 (human embryonic kidney cells, human airway epithelial cell line CFBE41o, Calu-3 human airway epithelial cell line | Curcumin (0-0.5, 1, 5, 10, 20, 30, 50 μM; 3, 7, 15, 25, 35, 45, 60 min) | Curcumin potentiates Δ1198-CFTR without cross-linking CFTR Polypeptides |
| Lipecka et al. (15) | HeLa cells stably transfected with WT-CFTR or ΔF508-CFTR, CALU-3 cells, or CF pancreatic epithelial cells CFPAC-1 | Curcumin (25 and 50 μM; 2, 4, and 16 h) | 1. ΔF508-CFTR is significantly delocalized toward the plasma membrane in curcumin-treated cells; 2. ΔF508-CFTR delocalization is accompanied by the reorganization of the K18 network |
| Goncalves et al. (16) | Normal (16HBE14o-normal human bronchial epithelial cell line) and DF508-CFTR human airway epithelial cell lines (CFBE41o-human bronchial epithelial, RCFTE29o-human tracheal epithelial) | Curcumin/poly(2-methyl-2-oxazoline-b-tetrahydrofuran-b-2-methyl-2-oxazoline; 0-50, 100, 150, 200, 250 μM; 2 h) | 1. The neutral amphiphilic copolymer of MeOx6-THF19-MeOx6 as a curcumin carrier facilitates the penetration of curcumin in normal and DF508-CFTR human airway epithelial cell lines; 2. Cur/TBCP2 formulation promotes the restoration of the expression of the CFTR protein in the plasma membrane; 3. Cur/TBCP2 formulation modifies the Cl– current at the membrane surface of F508del-CFTR cells |
| Harada et al. (17) | Chinese hamster ovary (CHO) cells, baby hamster kidney (BHK) cells | Curcumin (0 - 10, 20 μM; 24 h) | 1. Curcumin increases the CFTR level by downregulating calreticulin expression; 2. Curcumin decreases the mRNA expression and promoter activity of calreticulin; 3. Curcumin or CRT siRNA enhances the rescue of DF508 CFTR at 26 °C |
| Wang et al. (18) | HEK-293T cell | Curcumin (5 - 30 μM, 45 min) | 1. ATP-independent activation of G551D-CFTR and wild type CFTR channels by curcumin; 2. Curcumin activates CFTR channels that lack NBD2; 3. Gating properties of curcumin-activated channels; curcumin increases the opening rates of G551D-CFTR channels and the NBD2 deletion mutants; 4. Curcumin activation of 1198-CFTR channels is inhibited by ATP binding to NBD |
| Berger et al. (19) | Human airway epithelia (COS-7 cells) | Curcumin (10, 20, 30, 40, 50 μM; 3, 6, 18 h) | 1. Curcumin inhibits PKA activity; 2. Curcumin stimulates CFTR-F508; 3. Curcumin increases channel opening and slows channel closing; 4. Curcumin acutely increases Cl– current in differentiated non-CF airway epithelia |
| Dragomir et al. (20) | BHK cell lines transfected with DF508-CFTR, cystic fibrosis human bronchial epithelial cells CFBE41o, and nasal epithelial cells were isolated from CF patients, homozygous for the DF508-mutations | Curcumin (5, 10 μM; 24 h) | 1. Curcumin does not significantly affect the intracellular chloride concentrations in cells; 2. Curcumin does not cause a noticeable shift of DF508-CFTR to the plasma membrane; |
| Egan et al. (21) | Mice homozygous for F508 | Curcumin (45 mg/kg daily for 3 days) | Curcumin leads to a correction of ΔF508 CFTR and increased rates of survival |
| Song et al. (22) | Wild type and CF mice | Curcumin (45 mg/kg daily for 3 days) | Curcumin was unable to produce functional correction of ΔF508-CFTR processing in epithelial cells in culture models and CF mice |
| Cartiera et al. (23) | Gene-targeted mice homozygous for the ΔF508 mutation | Poly lactic-co-glycolic acid (PLGA) nanoparticles encapsulating curcumin (3.75 mg , for 4 days) | Nanoparticles encapsulating curcumin enhances the effects of curcumin as compared to delivery of nonencapsulated curcumin |
The cellular models and animals used in these studies included Chinese hamster ovary (CHO) cells (17), baby hamster kidney (BHK) cells (17, 20), COS-7 human airway epithelia (19), the Calu-3 human airway epithelial cell line (14, 15), CFBE41o cystic fibrosis human bronchial epithelial cells (13, 14, 16, 20), nasal epithelial cells (20), RCFTE29o- human tracheal epithelia (16), HEK-293T cells (14, 18), HeLa cells (15), and CFPAC-1 cystic fibrosis pancreatic epithelial cells (15), as well as wild type and CF mice (21-23). The mice weighed from 20 to 25 g.
Regarding the dosage and duration of curcumin use, the in vitro studies utilized 0 to 60 μM for 1 minute to 24 hours, whereas the in vitro studies applied 45 mg/kg of curcumin for 3 days.
3.3. Bias in the Studies
Quality of evidence was assessed with SYRCLE’s RoB tool (24) for animal studies. Two authors (E.F and F.F.R) categorized each item as “yes,” “no,” or “unclear” for each article. To assess the quality of these studies, information about allocation, randomization, and blinding were reviewed (Table 2).
| Type of Bias | Egan et al. (21) | Song et al. (22) | Cartiera et al. (23) |
|---|---|---|---|
| 1. Selection bias: Sequence generation | Y | Y | Y |
| 2. Selection bias: Baseline characteristics | Y | Y | Y |
| 3. Selection bias: Allocation concealment | Unclear | Unclear | Unclear |
| 4. Performance bias: Random housing | N | N | N |
| 5. Performance bias: Blinding | N | N | N |
| 6. Detection bias: Random outcome assessment | N | N | N |
| 7. Detection bias Blinding | N | N | N |
| 8. Attrition bias: Incomplete outcome data | Y | N | N |
| 9. Reporting bias: Selective outcome reporting | Y | Y | Y |
| 10. Other: Other sources of bias |
3.4. Main Results of the Studies
The main results of the articles are summarized in Table 1 and categorized as follows:
3.4.1. In Vitro Studies
A total of 8 studies analyzed the action of curcumin in vitro. Six of them showed that curcumin influences channel function through K18 network reorganization, calreticulin expression downregulation, CFTR phosphorylation, ATP adenosine triphosphate binding and dimerization of the 2 nucleotide-binding domains (NBDs), and activation of CFTR Channels without cross-linking CFTR polypeptides, thereby correcting the mutation in the ΔF508 CFTR protein.
Only 1 study (13) indicated that curcumin inhibits TLR2 through its antioxidant and anti-inflammatory activity. Curcumin could suppress the TLR2 gene and protein expression in a CF bronchial epithelial cell line (CFBE41o-cells). This performance was accelerated by the oxidative and proteasome degradation of specificity protein 1 (SP1), which is essential for the upregulation of TLR2 expression.
3.4.2. In Vivo Studies
Regarding the correction of the defective ΔF508-CFTR gene in vivo, 3 studies have been performed in CF mice. Of these, 2 reported channel correction with curcumin (21, 22), while the other found contrasting results (23). It should be noted that 2 studies used curcumin powder, while the other used curcumin nanocapsules.
4. Conclusions
Inflammation and lack of CFTR protein and/or function in CF patients lead to complications such as shortness of breath, inadequate and abnormal growth, diarrhea, constipation, and liver disease, for which there is no definitive cure (5, 25). Our increased understanding of the mechanisms that cause the disease and the function of natural compounds tested in vivo and in vitro has led to the development of novel agents for the treatment of CF. The main basis of treatment is now complemented by antibiotic regimens, anti-inflammatory agents, and new approaches that improve mucosal secretion (26-28). Therapies are currently targeting defects in the transport of major ions present in CF patients’ airways and include small molecular factors that restore function in regulating the membrane conduction of the mutated CFTR protein.
Researchers have shown that a novel treatment option is curcumin, an herbal antioxidant and anti-inflammatory drug that corrects electrolyte abnormalities (8, 21, 28). Several studies explored the contributions of curcumin to preventing CF complications. However, they reported contradictory results. Prior studies have noted the effects of curcumin on ion channels and pumps (10, 29), highlighting its potential effect on managing various ion channel conditions.
The findings of our review show that the current evidence is heterogeneous in all aspects of the study methodology.
Berger et al. (19) demonstrated that exposure of CF human airway epithelial cells to curcumin increased the activity of both wild-type and ΔF508 channels in the presence of ATP and PKA (protein kinase A) (protein kinase C) phosphorylation of CFTR. Regarding the direct effect of curcumin on PKA, it was reported that curcumin inhibited PKA phosphorylation of CFTR at a dose of 50 μg. Moreover, the administration of curcumin slowly increased the chloride current in non-CF epithelia but failed to alter the current in CF epithelia.
Similarly, Harada et al. (17) determined that curcumin did not enhance ΔF508 CFTR expression. However, in the presence of chaperone calreticulin (CRT) siRNA when cells were incubated at 26°C, it increased the activity of ΔF508 CFTR and resulted in a significant iodide efflux compared with the control.
Dragomir et al. (20) tested the effect of curcumin on cultured CF airway epithelial cells and nasal epithelial cells isolated from CF patients. In contrast to previous studies, their study concluded that curcumin did not significantly affect intracellular chloride concentrations.
Wang et al. (18) demonstrated that curcumin activated mutant CFTR channels independent of ATP binding and dimerization of the 2 NBDs. Moreover, Bernard et al. (14) reported that 1 derivative of curcumin could potentiate the G551D-CFTR channels and WT-CFTR, which had no cross-linking activity. In fact, it has been reported that a curcumin derivative, which has no cross-linking activity, can amplify G551D-CFTR channels and WT-CFTR channels.
Lipecka et al. (15) showed that remodeling in keratin 18 and increased K18 Ser52 phosphorylation in curcumin-treated cells caused the delivery of ΔF508-CFTR to the plasma membrane.
Furthermore, 1 study evaluated the action of curcumin dissolved in a copolymer consisting of methyl-2-oxazoline (MeOx) and tetrahydrofuran (THF; MeOx6-THF19-MeOx6; TBCP2) in DF508-CFTR cell lines, revealing that curcumin’s solubility and effect were improved upon administration of the curcumin/TBCP2 formulation. Three studies analyzed the function of curcumin in correcting the defective ΔF508- CFTR protein in vivo. Egan et al. (21) evaluated the action of curcumin by oral gavage to mice with the ΔF508 mutation (ΔF508 CF mice). They observed that curcumin led to adequate correction of ΔF508 CFTR, normalizing the level of epithelial sodium transport and increasing the rates of survival.
Song et al. (22) replicated the study of Egan et al. but could not prove the effect of curcumin on improving the function of defective F508-CFTR processing in transfected cells, native airway cells, and mutant mice. The cause of conflicting observations in different groups is still unclear. One of these may be the genetic differences in mice used in the studies, which should be considered before any clinical trial.
Cartiera et al. (23) showed upon oral administration of curcumin nanoparticles to CF mice, higher bioavailability of curcumin was achieved, leading to better correction of CF defects.
4.1. Strengths and Limitations
A key strength of the present study was its evaluation of both animal and cellular models of CF. However, this work encountered several limitations. Mostly, we discovered significant heterogeneity when comparing the available evidence. This heterogeneity made statistical analysis impossible in our systematic review.
In general, the results of this systematic review indicate the beneficial effects of curcumin in CF via different mechanisms. Still, there are numerous gaps in the basic science related to this topic. Given that few studies have been conducted in this field, this systematic review suggests that more studies be conducted to facilitate generalization to human studies.