1. Background
Transient tachypnea of the newborn (TTN), also known as newborn wet lung, is a benign, self-limited breathing condition that can present in infants of any gestational age (1). It is caused by a delay in the clearance of the fetal lung fluid after birth, which leads to tachypnea (a respiratory rate greater than 60 breaths per minute) and respiratory distress (grunting, flaring of the nostrils, and intercostal retraction). The incidence of TTN is approximately 10% in late preterm infants and less than 5% in term infants (2, 3). Although TTN is usually a self-limiting condition, malignant TTN can cause newborns to develop persistent pulmonary hypertension or pneumothorax requiring mechanical ventilation.
Lung ultrasound (LUS) is a very convenient, radiation-free technique available at the bedside. It has been increasingly applied in the diagnosis and management of neonatal respiratory disorders, including respiratory distress syndrome (RDS), pneumonia, and pneumothorax (4, 5). Recently, LUS has been used for lung liquid clearance management after NICU admission (6). A meta-analysis showed that lung ultrasound is an extremely accurate diagnostic tool that may be a superior alternative to chest X-rays in diagnosing TTN. Lung ultrasound (LUS) has a pooled sensitivity of 0.98 and a specificity of 0.99 (7). According to previous studies, the ultrasound finding of a severe type of TTN primarily manifests as a compact B-line profile or even “white lung”, and the mild type of TTN primarily manifests as alveolar-interstitial syndrome (AIS).
In recent years, LUS has gained broader application, as it has been used to predict that patients, from the population of term and late preterm infants born by cesarean section, with transient neonatal tachypnea or respiratory distress syndrome will require an LUS check after NICU admission (8). Lung ultrasonography is becoming a point-of-care method to guide respiratory support in critical care infants. The LUS score correlates well with the oxygenation status in both term and preterm neonates, and it shows accuracy in predicting surfactant administration in preterm newborns (9).
However, only a few studies have been performed using LUS scores to assess the severity of TTN. Additionally, there are few previous studies using the LUS to predict the need for respiratory support in newborns with TTN.
2. Objectives
In this prospective study, we evaluated the clinical capability of LUS to predict the severity of TTN and any respiratory support need.
3. Methods
3.1. Subjects
From July 2021 to September 2021, lung ultrasonography was performed on neonates admitted to the Department of Neonatology of the authors’ hospital. The inclusion criteria, which were also the diagnostic criteria for TTN, were as follows: (1) the infants showed clinical manifestations of respiratory distress, such as tachypnea (respiratory rate > 60 breaths per minute), nasal flaring, grunting, and showing retractions beginning within 6 hours of delivery (10); (2) infants without suspected pneumonia. The diagnosis of pneumonia was as follows: an axilla temperature of > 37.5°C or < 36.5°C, abnormal laboratory parameters (C-reactive protein (CRP) > 10 mg/L, leukocytosis (white cell count > 20 × 109/L) or leucopenia (white cell count < 5 × 109/L) (11), isolation of a pathogenic microorganism in the airway aspirate; (3) LUS examination shows alveolar-interstitial syndrome (AIS), no lung consolidation and pleural effusion (10).
The exclusion criteria were as follows: (1) infants with congenital heart disease, except PDA and patent foramen ovale, confirmed by a cardiac ultrasound; (2) infants with definite congenital lung disorders, including congenital cystic adenomatoid malformation (CCAM), or pulmonary sequestration; and (3) infants with RDS, pneumothorax and pulmonary hemorrhage confirmed by chest radiography and clinical manifestations.
This study was reviewed by the author’s hospital’s ethics committee (No. 2021-7-28). Informed consent was provided by each infant’s immediate family or guardian prior to the examination. All methods were performed in accordance with the Declaration of Helsinki.
3.2. Respiratory Support and Measurement
In our institution, infants who displayed clinical symptoms of respiratory distress, including nasal flaring, tachypnea, retractions, grunting and cyanosis, were transferred to the NICU. During the two hours after birth, arterial blood gas, blood culture, and CRP were determined using routine analytical methods. If retractions, grunting, hypercarbia (PaCO2 > 60 mm Hg), or hypoxia (SpO2 < 90%) (10) were evident, we commenced noninvasive ventilation (NIV), including humidified high-flow oxygen delivered by nasal cannulae (HHHFNC) or nasal intermittent positive pressure ventilation (NIPPV). High flow has gained popularity because of its ease of use, reduced nasal injury, and increased comfort. According to our NICU respiratory support guidelines, term and late preterm infants who develop mild respiratory distress will receive HHHFNC, while neonates with significant respiratory distress will require NIPPV. The degree of respiratory distress was evaluated by a neonatologist and according to the clinical symptoms.
Treatment with HHHFNC is the preferred means of noninvasive respiratory support, providing an initial gas flow of 6 to 8 liters per minute using the Optiflow Junior device (Fisher and Paykel Healthcare). Neonatal-sized nasal prongs were used to provide airway pressure. The percentage of inspired oxygen (FiO2) was altered to retain an oxygen saturation level (SpO2) between 90% and 95% in late preterm infants [born at 34 (0/7) - 36 (6/7) weeks gestation] and > 94% in term infants using a pulse oximeter (12) (the PHILIPS IntelliVue MX550 Bedside Patient Monitoring System, Germany). Newborns with continued respiratory distress on HHHFNC required continuous oxyhemoglobin saturation monitoring to assess the need for NIPPV, which would be provided if the SpO2 was < 90% (10).
NIPPV respiratory support was delivered using the ''Fabian + NCPAP'' evolution ventilator” (Hirzel, Switzerland) via nasal prongs. The ventilator was set with a positive peak inspiratory pressure (PIP) of 18 to 20 cm H2O until we obtained good air entry, a positive end expiratory pressure (PEEP) of 5 to 8 cm H2O, and a breath rate of 30 to 40 breaths per minute. We adjusted the FiO2 to maintain the targeted oxygen saturation mentioned above. PIP was increased in steps of 2 cm H2O each time until we obtained good blood gas results, including pH: 7.35 - 7.45, PaO2: 60 - 80 mm Hg, and PaCO2: 35 - 45 mm Hg (13).
We defined HHHFNC and NIPPV failure as the infant receiving maximum support settings (a flow rate of 8 L/min in the HHHFNC group or PEEP pressure of 7 cm H2O in the NIPPV group) and having more than one of the following criteria: (1) the development of respiratory acidosis (blood gas showing a pH < 7.2 and PaCO2 > 60 mmHg on noninvasive ventilation); (2) hypoxemia (FiO2 requirement > 0.40 to achieve the target oxygen saturation); (3) frequent desaturation (SpO2 ≤ 90%): ≥ 3 episodes per hour and not responding to increased ventilator support settings (14-16). In our clinical practice, we do not have a maximum value for the PIP in term and late preterm infants. PIP is adjusted to achieve adequate chest expansion and equal and good breath sounds. We used synchronized intermittent mandatory ventilation (SIMV) as the mechanical ventilation (MV) mode. The ventilator settings were adjusted according to the infant’s oxygenation, chest wall movement, breath sounds, and respiratory efforts along with arterial blood gases.
3.3. Lung Ultrasounds
An Esaote ultrasonic diagnostic apparatus (My Lab 700) and linear array probe set at a frequency of 10 - 14 MHz were used. The infants were placed in prone, lateral or supine positions. With the anterior and posterior axillary lines as the boundary, each side of the lung was separated into three different scan areas: the anterior, lateral and posterior areas. Using the connection line between the nipples, we divided each lung into upper and lower parts, resulting in a total of 12 regions on either side of the lungs. The probe was placed perpendicularly or parallel to the ribs when scanning each lung area. Considering that there is inter-observer variance in sonographers’ skills, the ultrasound scans were performed by only one operator.
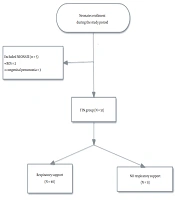
Lung ultrasound (LUS) was performed as soon as possible after NICU admission once the infant was stable. The LUS score was calculated based upon the 6 LUS scanning areas for each side (4, 6): lung sliding signs with A line or a small amount of B line, 0 points; more than three B lines, spacing between B lines, no fusion, 1 point; confluent B-line defined as the entire intercostal space filled with B-lines and it is difficult to distinguish from each other between two acoustic shadows of the ribs, 2 points; and compact B-lines and this type of B-line may cause the acoustic shadow of the ribs to disappear within the entire scanning zone, 3 points. Each region was rated with the most serious performance. The LUS was the sum of the scores of each region, and the highest score of the 12 segments was 36 points (Figure 1).
A, Ultrasound score: 0 points, A line, a small amount of B line; B, Ultrasound score: 1 point, more than three B lines, space between B lines; C, Ultrasound score: 2 points, confluent B-line (B-line fusion) and some A lines are present; D, Ultrasound score: 3 points, compact B-lines, a white lung is present within each scanning zone, without lung consolidation.
3.4. Data Collection
All data were obtained from clinical notes after official approval was obtained from the hospital administration. The requirements for patient consent was waived. The demographic data, including gestational age, birth weight, sex, mode of delivery, and ventilator settings, were documented. According to our institution’s routine guidelines, assessments of arterial blood gas (ABG) results were performed 1 h after the ventilator support response (the ABL90 FLEX PLUS blood gas analyzer, Radiometer Medical ApS, Denmark). All data were anonymous and used only for research purposes. We collected the LUS score within 1 hour of admission. We also collected the ventilator setting [fraction of inspired oxygen (FiO2), mean airway pressure (MAP), and positive end-expiratory pressure (PEEP)] and oxygenation index (OI) calculated as follow:
3.5. Statistical Methods
All neonates were divided into two groups depending on the ventilator required after enrollment: an NIV group who received NIV, an MV group who received MV and an NRS group who did not require respiratory support. Normally distributed data are expressed as the mean [standard deviation (SD)]. Proportions are shown as numbers (percentages) and were compared using the chi-square test or Fisher's exact test. Multiple means were compared using one-way analysis of variance (ANOVA), with the post hoc least significant difference (LSD) method used to test for differences between each pair of groups. One-way analysis of variance (ANOVA) and LSD tests using 100 bootstrap resampling results showed considerable variation. Therefore, 10 items of data were randomly selected from the 100 replicates (1000 bootstrap resampling replicates). Analysis of the correlation between the ABG results and LUS scores was performed by computing Pearson's correlation coefficient (r) and P value; the received operating characteristics (ROC) curve was plotted to illustrate the accuracy in recognizing patients with severe TTN. The statistical analysis was performed using SPSS version 20.0 and Medcalc software version 19.6.1. P values lower than 0.05 were regarded as significant.
4. Results
4.1. Basic Demographic Data
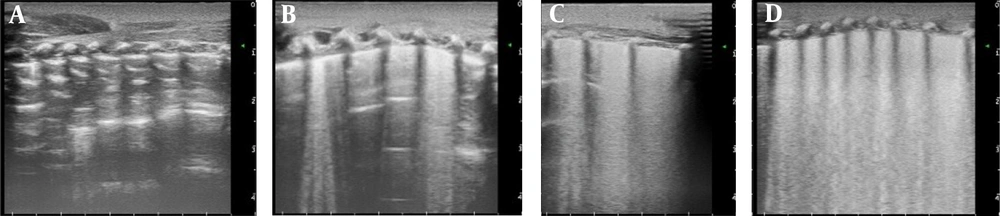
There were a total of 51 patients who were eligible for this study. Figure 2 describes the enrollment process. All infants received immediate LUS checks within two hours of enrollment as our study protocol described. Five infants were excluded for various reasons: two infants clinically developed RDS after enrollment, while three had congenital pneumonia after enrollment. Finally, we continued the study with 51 infants. All patients were weaned from noninvasive ventilation, and none received surfactant treatment. Among them, 17 (33.3%) were females, and 34 (66.7%) were males. The mean gestational age was 35.4 ± 2.5 weeks, and the mean birth weight was 2.42 ± 0.55 kg. The three groups were comparable in demographic and clinical characteristics. No significant differences were observed between the two groups regarding the basic data. The mean age at the first LUS examination was 1.22 ± 0.81 hours on admission. The period from birth to the ultrasound check showed a significant difference between groups, and there was a longer period of time in the MV group than in the NIV and NRS groups. The infants in the MV group were in critical condition and needed extra care (shown in Table 1).
| Variables | NRS (N = 11) | NIV (N = 35) | MV (N = 5) | Statistic | P Value |
|---|---|---|---|---|---|
| Gestational age (weeks) | 0.55 | 0.761 b | |||
| ≥ 37 | 3 (27.3) | 6 (17.1) | 1 (20.0) | ||
| 34 ~ 36+6 | 8 (72.7) | 29 (82.9) | 4 (80.0) | ||
| Birth weight (kg) | 2.67 ± 0.60 | 2.34 ± 0.54 | 2.40 ± 0.39 | 1.61 | 0.211 |
| Sex, male | 10 (90.9) | 20 (57.1) | 4 (80.0) | 4.74 | 0.094 |
| Mode of delivery | 0.85 | 0.652 | |||
| C-section | 3 (27.3) | 15 (42.9) | 2 (40.0) | ||
| Vaginal delivery | 8 (72.7) | 20 (57.1) | 3 (60.0) | ||
| Apgar score (min) | |||||
| 1 | 9.7 ± 0.6 | 9.4 ± 0.9 | 10 ± 0 | 1.22 | 0.303 |
| 5 | 10 ± 0 | 9.7 ± 0.6 | 10 ± 0 | 1.63 | 0.206 |
| 10 | 10 ± 0 | 9.9 ± 0.5 | 10 ± 0 | 1.11 | 0.338 |
| Time from birth to first LUS check | 1.29 ± 0.72 | 1.05 ± 0.76 | 2.23 ± 0.39 b, c | 5.82 | 0.005 |
Abbreviation: NRS, nonrespiratory support; NIV, noninvasive ventilation; MV, mechanical ventilation.
a Quantitative data are expressed as mean ± SD and qualitative data are expressed as No. (%).
bSignificantly different (P < 0.05) compared to the NIV group.
c Significantly different (P < 0.05) compared to the NRS group.
4.2. LUS Score Comparison
Significant differences (P < 0.05) were revealed among the three groups in the left anterior area, right lateral, left lateral area and total LUS scores. The LUS scores in the NRS group were lower than those in the other groups. Two-two comparisons of all the means were performed using LSD pairwise comparison methods by comparing the patients with nonrespiratory patients. The infants with NIV and MV were more likely to have a higher LUS score (P < 0.05), and the LUS scores in the MV group were significantly higher than that in the NIV group. Furthermore, the LUS scores between the HHHFNC and NIPPV groups were also compared. The data showed significant differences between the two groups in the left anterior, right posterior, left posterior areas and total ultrasound scores. Infants in the MV group showed significantly higher LUS scores (shown in Tables 2 and 3).
| LUS Score | NRS (N = 11) | NIV (N = 35) | MV (N = 5) | F Value | P Value |
|---|---|---|---|---|---|
| Right anterior area | 1 ± 0.8 | 1.7 ± 1.4 | 1.8 ± 0.8 | 1.42 | 0.252 |
| Left anterior area | 1 ± 0.9 | 2 ± 1.2 b | 2.4 ± 1.1 b | 3.96 | 0.026 |
| Right lateral area | 1 ± 0.9 | 2.2 ± 1.1 b | 3.8 ± 0.4 b,c | 12.88 | < 0.001 |
| Left lateral area | 0.8 ± 0.8 | 2.3 ± 1.3 b | 3.6 ± 0.5 b,c | 11.11 | < 0.001 |
| Right Posterior area | 1.3 ± 1.4 | 2.9 ± 1.8 b | 4.4 ± 1.5 b | 6.41 | 0.003 |
| Left Posterior area | 1 ± 1.6 | 3 ± 1.7 b | 4.4 ± 1.5 b | 8.48 | 0.001 |
| Total ultrasound score | 6.1 ± 3.7 | 14.1 ± 7 | 20.4 ± 4.4 | 10.78 | < 0.001 |
a Values are expressed as mean ± SD.
b Significantly different (P < 0.05) compared to the NRS group.
c Significantly different (P < 0.05) compared to the NIV group.
| LUS Score | HHHFNC (N = 11) | NIPPV (N = 23) | t Value | P Value |
|---|---|---|---|---|
| Right anterior area | 1.4 ± 0.7 | 1.8 ± 1.6 | 0.91 | 0.371 |
| Left anterior area | 1.1 ± 0.8 | 2.5 ± 1.2 | 3.54 | 0.001 |
| Right lateral area | 1.6 ± 0.8 | 2.5 ± 1.2 | 2.10 | 0.044 |
| Left lateral area | 1.6 ± 1.7 | 2.6 ± 1 | 2.08 | 0.046 |
| Right Posterior area | 2 ± 1.5 | 3.5 ± 1.7 | 2.41 | 0.022 |
| Left Posterior area | 2 ± 1.5 | 3.4 ± 1.7 | 2.37 | 0.024 |
| Total ultrasound score | 9.7 ± 5.5 | 16.3 ± 6.8 | 2.79 | 0.009 |
aValues are expressed as mean ± SD.
4.3. Correlation Analysis
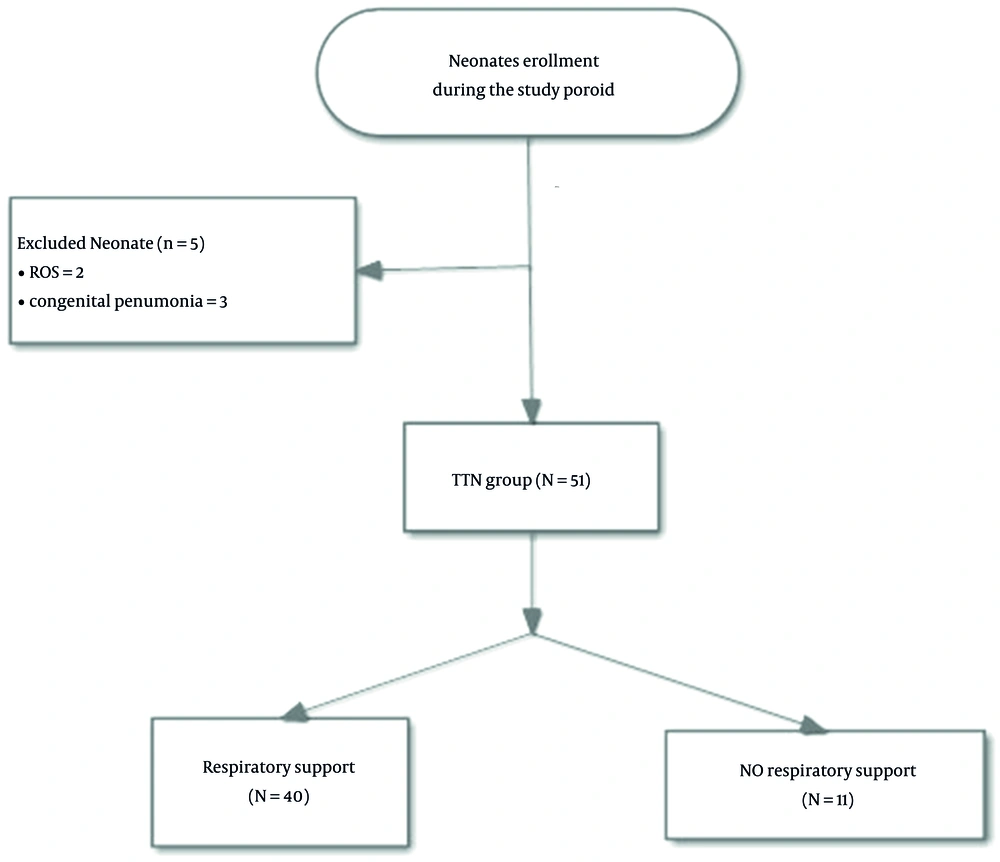
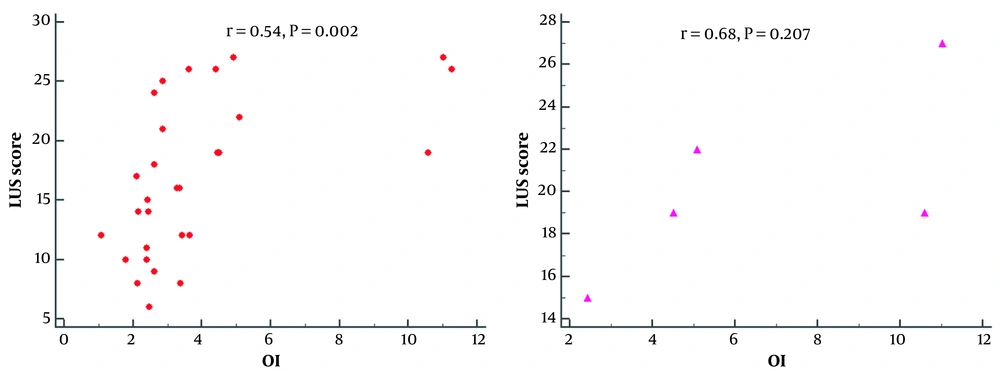
The lung ultrasound scores were negatively correlated with the PaO2 results (r = -0.25, P = 0.073), positively correlated with PaCO2 (r = 0.41, P = 0.003), and significantly correlated with SaO2 (r = 0.35, P = 0.015). A scatter plot of the relationship is shown in Figure 3. Further correlation analysis showed that the LUS scores correlated moderately with the oxygenation index (r = 0.54; P = 0.002) in the NIPPV group and (r = 0.68; P = 0.207) in the MV group (shown in Figure 4).
4.4. ROC Analysis
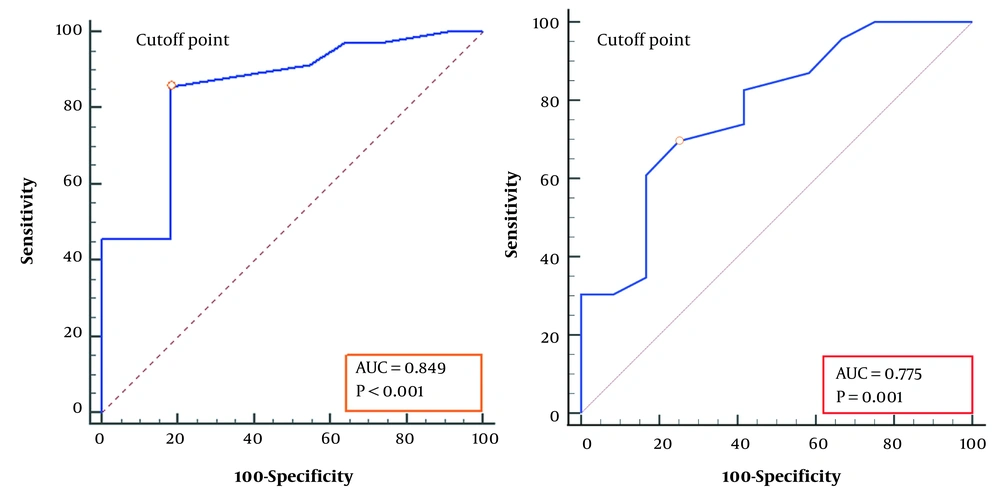
Received operating characteristics (ROC) analysis revealed that an LUS score of 6 (AUC = 0.85, P < 0.001) can predict the need for respiratory support in TTN infants with a sensitivity of 85.7%, a specificity of 81.8%, a positive predictive value of 93.7%, and a negative predictive value of 64.3%. LUS scores of 11 (AUC = 0.78, P < 0.001) predicted the need for NIPPV support with a sensitivity of 80.0%, a specificity of 78.3%, a positive predictive value of 84.2%, and a negative predictive value of 56.2% (shown in Figure 5).
Receiver operating characteristic analysis for the prediction of respiratory support; A, receiver operating characteristic curve for the prediction of respiratory support (NIPPV or MV) according to the cutoff values of the LUS scores (> 6, the sensitivity and specificity were 0.86 and 0.82), the control group included babies who did not need respiratory support; B, The curve indicated the prediction of infants with NIPPV support. The control was babies with HHHFNC, the cutoff value of the LUS score was > 11, and the sensitivity and specificity were 0.70 and 0.83, respectively.
5. Discussion
We demonstrated a high level of diagnostic accuracy using quantitative LUS scores for predicting the respiratory support needs of newborns with transient tachypnea. An LUS can be used to accurately predict the different respiratory support needed. The higher the score, the more powerful the support needed. These results were not influenced by the GA within the age range of the enrolled population.
Transient tachypnea of the newborn (TTN) is the frequent cause of respiratory distress syndrome in premature newborns. In recent decades, the preterm birth rate has increased, mostly due to a rise in late preterm births (17). A recent study showed that more than half of infants with TTN required respiratory support (18). Non-invasive or mechanical ventilation respiratory support may be administered to reduce respiratory distress during TTN. In addition, respiratory support might improve the clearance of lung liquid, reducing the effort required to breathe and therefore reducing respiratory distress (1). The meta-analysis showed insufficient evidence to establish the benefits and harms of noninvasive respiratory support in the management of newborn transient tachypnea. Continuous positive airway pressure (CPAP) remains the most noninvasive respiratory support in the NICU. In a meta-analysis including preterm infants requiring respiratory support, NIPPV proved to be more efficient than CPAP for reducing the need for intubation (19). As a result, an increasing number of doctors in our NICU favored NIPPV instead of CPAP in infants with respiratory distress. Therefore, we did include infants with CPAP respiratory support due to the limited utilization.
Ultrasonography is a safe, inexpensive and accurate diagnostic tool. It provides real-time, quick and minimally invasive information without significant biological hazards. Lung ultrasonography (LUS) has been successfully used to diagnose neonatal disease. A previous study showed that the sensitivity and specificity of LUS for the diagnosis of TTN were 76.7% and 100%, respectively. After transfer to the NICU, this intervention can be conducted, distinguishing it from other critical lung diseases and facilitating proper initial treatment. LUS has already become a point-care tool for identifying respiratory illness and providing intervention guides. However, these descriptive findings are more qualitative in content. In recent years, lung ultrasound has been widely used to diagnose neonatal lung diseases. B lines can reflect the degree of pulmonary interstitial edema, but the utility of lung ultrasound scores by counting the number of B-lines in the assessment of severity is controversial. Min Zhao proposed using the neonatal LUS score to predict extravascular lung water, and the results showed that the lung ultrasound score can semiquantitatively evaluate the extravascular lung water content (20).
The early-preterm neonate may suffer from various respiratory disorders due to different degrees of surfactant insufficiency. With the current study, this population was excluded because an LUS is more useful in infants (even late-preterm infants) than in more mature neonates > 37 weeks’ GA. This is likely because of the homogeneity of the preterm population, which is predominantly affected by TTPN. Infants with RDS or pneumonia were excluded from our study. Our aim was to verify whether an LUS was accurate enough for predicting in the higher GAs.
According to the previous LUS scoring systems, there are two methods of zoning, one that uses 12 zones and one that uses 6 zones. The 12-zone score was selected for this study. First, the infants in this study were late preterm babies, so the body surface area was limited. Second, previous studies revealed that the upper and lower areas showed different LUS phenomena in TTN (21). Each side of the lung was divided into three areas (anterior, lateral and posterior), and every area was also divided into upper and lower sections. Therefore, 12 zones were recorded. Each area was assigned a score ranging from 0 to 3 (0 = normal lung pattern with A line, lung sliding and less than three B lines, 3= extended consolidations, with poor lung aeration) (7). However, this LUS scoring system was used to assess lung aeration in neonates with RDS or bronchopulmonary dysplasia, so the authors did not assess posterior lung zones (22). Unlike diffuse inflammatory lung diseases, such as MAS or BPD, neonates with TTN always showed increases in their extravascular lung water content. The LUS scan excluded lung consolidation, so the previous lung scoring system was not useful in the assessment of TTN. We modified the previous LUS scoring systems because confluent B-lines and compact B-lines are commonly observed in infants with diffuse alveolar edema, and consolidation was not observed. In another previous study, the posterior zone was never scanned by the LUS protocol because the population was almost all early-preterm infants (20). In this study, the posterior zone was also scanned, providing more information about LUS scores.
In this prospective study, every lung area was scanned to assess the TTN severity of infants during NICU admission using quantitative LUS scores. Several LUS findings were utilized in the diagnosis of TTN in our study, such as confluent B-line, double lung point, compact B-line and white lung (7). Our study shows that LUS scores are correlated with severe conditions of TTN. Neonates with higher LUS scores required more advanced respiratory support. Li et al. (6) revealed that the LUS score after birth decreased with respiratory support. In comparison with the neonates in the control group, the LUS score of the TTN group was significantly higher. Our study showed that affected neonates with higher scores always developed respiratory distress due to the accumulation of fluid in the lungs and needed assisted respiratory support. It also displayed an early positive correlation between LUS scores and PaCO2 of the ABG results and a negative correlation between PaO2, SaO2 and the scores; however, the correlation was not significant because the sample size was not adequate. Our study indicated that infants with higher LUS scores experienced severe TTN and needed appropriate respiratory support. If the scores drastically increased, mechanical ventilation should probably be provided. Another study showed that LUS scores had a greater decrease after birth in the infants that needed TTN in comparison with the control group. In infants with TTN, the scores were significantly associated with the respiratory severity scores (RSSs) (6). In another study, quantitative ultrasound texture analysis of the fetal lung was conducted. It could predict neonatal respiratory morbidity in preterm infants, the accuracy of which was almost 86.5%. Furthermore, its positive and negative likelihood ratios were 6.5 and 0.3, respectively (23).
TTN is a neonatal disease related to multiple risk factors, and it is mainly attributed to fluid clearance failure and epithelial Na+ channel abnormalities (1). Some infants with hypoxemia and respiratory failure have increased the need for ventilation support in the NICU (18). Several studies were conducted to identify some clues that may help to predict the severity of the disease and need for different respiratory support. Kahvecioglu et al. (18) demonstrated that a positive correlation was found between the arterial blood gas result (pH < 7.30), ratio of PaO2/% inspired O2 < 1.2 and the need for ventilator support. In our study, we revealed that the LUS score was significantly correlated with the severity of clinical respiratory conditions after admission. In this study, a new LUS scoring system was proposed. The ROC analysis suggested that this new scoring system could be better for evaluating the severity of TTN. Infants with a higher LUS score (> 6 units) might be provided noninvasive ventilation support; moreover, an LUS score over 11 units might be a good predictor of the need to provide NIPPV ventilation. Because few infants were included in the MV group, how to use the LUS score to predict mechanical ventilation was not analyzed.
In this study, only one doctor conducted the LUS procedure, and different operators’ biases were minimized. However, there are also some limitations in this research. First, the sample size was smaller than that in other studies. For a more significant result, we need to include more infants who meet the criteria. Second, the double-blind condition was not conducted in this study, as the physician who performed the LUS scan recognized the neonatal clinical condition and mode of respiratory support. Nonetheless, if the operator had knowledge of the relevant clinical data and respiratory modes, the LUS score was likely to be misestimated by experimenter bias. Finally, TTN was diagnosed by typical clinical indications and the result of ultrasound scan and was not diagnosed pathologically, as it is difficult to find rare conditions, such as alveolar capillary dysplasia with misalignment of pulmonary veins, alveolar growth disorders, etc., which are usually evaluated by high-resolution CT.
LUS is a dynamic, point-of-care tool in addition to the available tools that dramatically decrease neonates’ exposure to radiation. It offers increased sensitivity in capturing evidence of pulmonary edema and evaluating the clinical effectiveness of follow-up. In this study, the LUS check results on admission can reveal the respiratory conditions of patients with TTN disease accurately. The LUS will be a good predictor for the decision on the respiratory mode needed.