1. Context
The increasing number of factories and vehicles has accelerated the release of inhalable environmental contaminants, such as heavy metals, which can cause serious respiratory diseases and severe health problems (1). Although zinc is a potential hazardous heavy metal, it is also a beneficial micronutrient playing critical roles in the body (2). Heavy metals inhaled by the respiratory system may facilitate the development of respiratory diseases such as asthma depending on their dose and nature (2, 3). Asthma is an inflammatory disease of the respiratory tract caused by exaggerated responses to immunologic and non-immunologic stimuli (3). Some asthma symptoms include wheezing, coughing, and dyspnea, which are known as the asthma triad (3). Asthma diagnosis is usually based on clinical symptoms, response to treatment, and spirometry findings (4). Asthma occurrence has been increasing in developing countries in recent years (4).
Lead is as another potentially hazardous heavy metal that can activate T helper lymphocytes, stimulating the respiratory tract and triggering allergic diseases (5, 6). In addition, lead can activate T helper 2 (Th2) lymphocytes, induce interleukin 4 (IL-4) production and, subsequently, boost IgE production, which is an indicator of allergic diseases. Allergens further induce respiratory tract contraction by modulating Th2 lymphocytes, inducing IgE production, affecting mast cells, and inducing the release of inflammatory mediators such as histamine. Furthermore, activated Th2 lymphocytes amplify respiratory tract contraction by producing IL-5 and inducing eosinophils to release other mediators (4, 6). Lead storage in the body is around 150 - 400 mg in an adult, and blood lead level (BLL) reaches around 25 µg/dL (1, 2). Elevated BLL to 70 µg/dL is usually accompanied with clinical symptoms (3-5).
Lead and other heavy metals are considered as serious environmental and occupational hazards (4). Lead performs no beneficial role in the body but inflicts harms to various body organs. Lead-induced detrimental effects include hypertension, nephrotoxicity, cardiovascular disorders, hemoglobinopathies, anemia, and memory loss (7, 8). Evidence suggests the relationship between lead and asthma; however, such a potential relationship has not received due research attention (9, 10). Lead can be measured in various biological samples such as blood, urine, and saliva (11).
In short children, zinc can boost the height. On the other hand, zinc alleviates common childhood infections such as diarrhea and pneumonia. The zinc content of the body ranges from 5.1 to 5.2 grams, most of which is accumulated in muscles, bones, and the liver, while lesser amounts present in nails, skin, and hair. Zinc is a vital element in the structure of more than 300 enzymes within the body. Zinc deficiency can trigger multiple metabolic disorders and organ dysfunction, and negatively affect growth and development (12, 13). Zinc deficiency may result in growth retardation, short statue, delayed puberty, delayed wound healing, and hair loss (12, 13). Daily zinc requirement is around 10 - 12 mg, which is required for growth and development (12, 13). Vegetarians, individuals with renal/hepatic failure, diabetics, alcohol abusers, and lactating/pregnant women are prone to zinc deficiency (12, 13). Olfactory and taste problems, nail and skin abnormalities, defects in vision, and memory loss are some of the complications of zinc deficiency (12, 13).
Zinc deficiency has been found associated with respiratory diseases such as asthma (12), as well as with cold-like symptoms, diarrhea, immunodeficiencies, and childhood growth abnormalities (13). This nutrient is available in red meat, chicken meat, shrimp, fish, beans, and dairy products (14). Adequate zinc levels can prevent asthma; however, this notion needs to be verified (15).
No comprehensive systematic reviews and meta-analyses have investigated the role of lead and zinc in asthma development. Meta-analyses combine the results of other studies to validate their outcomes (16, 17). Regarding the presence of numerous studies on asthma around the world, there is a need to merge these findings to obtain conclusive outcomes.
2. Objectives
This study aimed to compare the impacts of lead and zinc on the development of asthma worldwide regarding separate different geographical locations, age groups, disease subtypes, and risk factors. A meta-analysis study was performed to compile the data from multiple studies on asthma around the world and to obtain quantifiable and conclusive outcomes.
3. Methods
The research protocol included four steps: designing the search strategy, collection and systematic screening of the studies, screening for inclusion and exclusion criteria, and, finally, statistical analysis of the data. To avoid bias during data analysis, all the above-mentioned steps were independently performed by two researchers.
The first step included conducting an online search in international databases, such as Web of science, Cochrane Library, Google, PubMed, Scopus, and Google scholar search engine to reach studies published between 2000 and 2018. The search was independently conducted by two researchers using Mesh keywords and Boolean operators (AND, OR, NOT) (i.e., “asthma AND lead”, and “asthma AND zinc sulfate”). The search results were then screened to check their relevance to the research question.
All articles with titles containing the keywords “lead and asthma” and “zinc and asthma” were downloaded. Studies investigating asthma complications and non-original studies, as well as letters to editors, short communications, congress abstracts, and unpublished data were excluded.
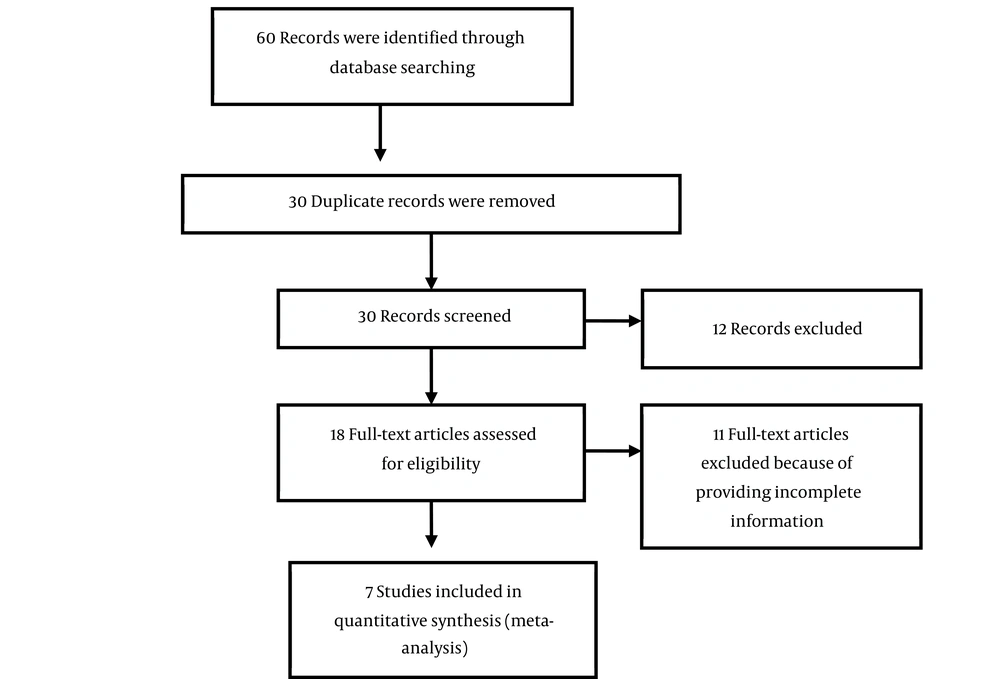
A checklist was used for the primary screening of the articles. The screening process was independently completed by two researchers. Using the aforementioned criteria, 60 related articles were found, whose titles and abstracts were separately assessed by the same two researchers. If the study was relevant, its full text was retrieved and studied. By reading the full texts, six duplicate articles were excluded; 24 additional studies were excluded due to irrelevancy, and 18 articles were excluded due to having inadequate data. Finally, seven studies were selected for the meta-analysis.
The extracted data were coded in STATA version 14 software, including first authors’ names, the date and location of the studies, titles, sample sizes, the number of boys and girls, the relationship between asthma and lead/zinc levels, as well as asthma-related factors. These factors were analyzed for different age groups and according to various risk factors, sample sizes, and the localization of code subgroups.
The final parameters recorded in the checklist were the sample size, study’s date of conduction, relationships between lead/zinc and asthma, and the factors influencing asthma based on the sample size, age groups, and risk factors. Studies having reported the above factors were included in meta-analysis. Based on the above criteria, seven studies were selected for meta-analysis, and their full texts were thoroughly reviewed.
The relationship between either lead or zinc and asthma was estimated based on gender, sample size, and the factors influencing asthma. The variance of each study was calculated utilizing normal distribution, and mean weight was applied to determine the impacts of both lead and zinc on childhood asthma. Each study was assigned with a weight that was inversely related to its variance. Regarding the large differences between the effects of lead and zinc on asthma in different studies (i.e., high heterogeneity among the studies) as well as a statistically significant heterogeneity index (I2), the random effects model was employed for meta-analysis. The I2 index was considered to estimate heterogeneity among the studies. The I2 index is a quantitative parameter utilized as a surrogate to the probability index to evaluate variance among studies with statistical heterogeneity (18, 19). The heterogeneity index in this study was calculated as 93.5%, which is considered a high heterogeneity index. Meta-regression was performed to compare the impacts of lead and zinc on asthma worldwide, in different genders, based on different sample sizes, factors influencing asthma, and years of publication. Meta-regression was also used to identify the reasons for heterogeneity among the studies. The data were analyzed using STATA (version 14) software. Figure 1 displays Flowchart of the systematic review and meta-analysis process.
4. Results
The selected studies had been published between 2000 and 2018. The total sample size was 6353, giving a mean of 907 individuals per study. In all analyzed studies (i.e., those assessing the relationship between asthma and lead/zinc sulfate), the subjects were recruited using checklists. All studies reported asthma prevalence, blood lead percentage, BLL grades, ethnicity, gender, age groups, the mean level of zinc, and mean FEV in asthma patients.
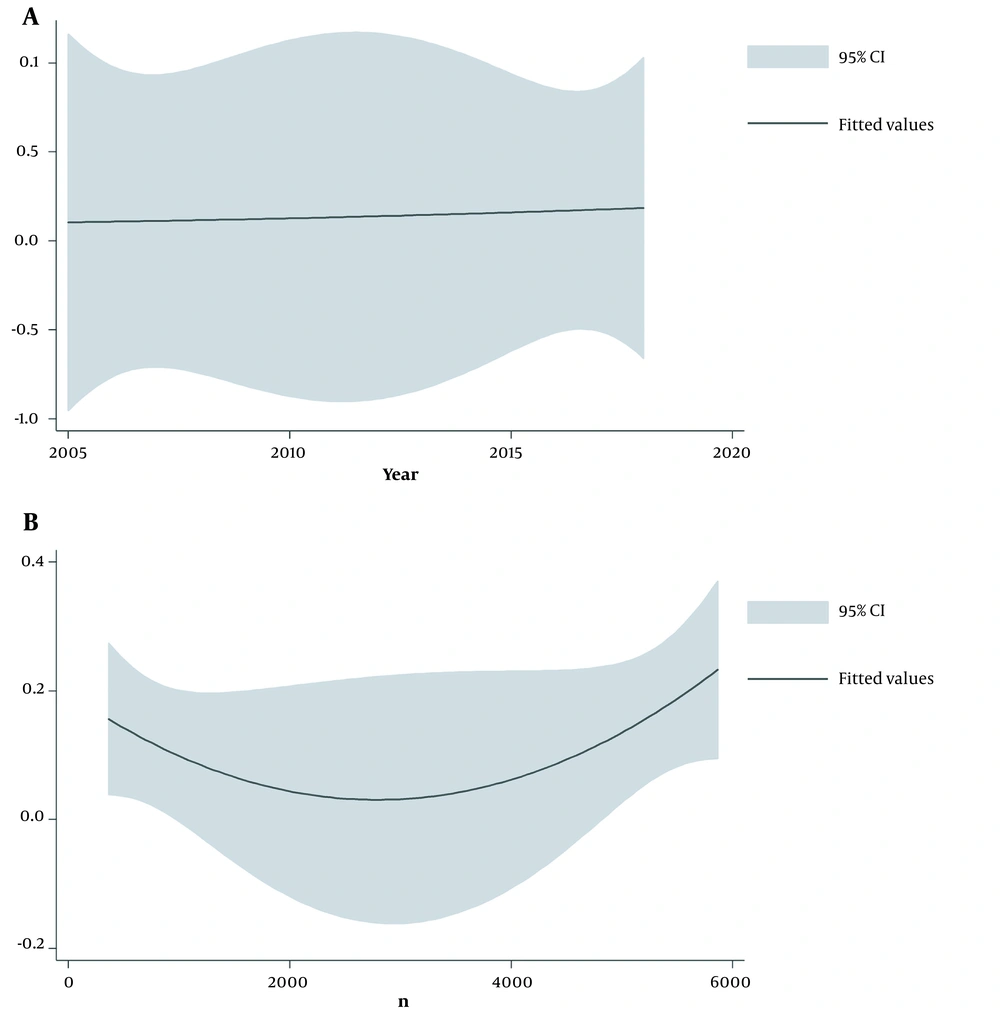
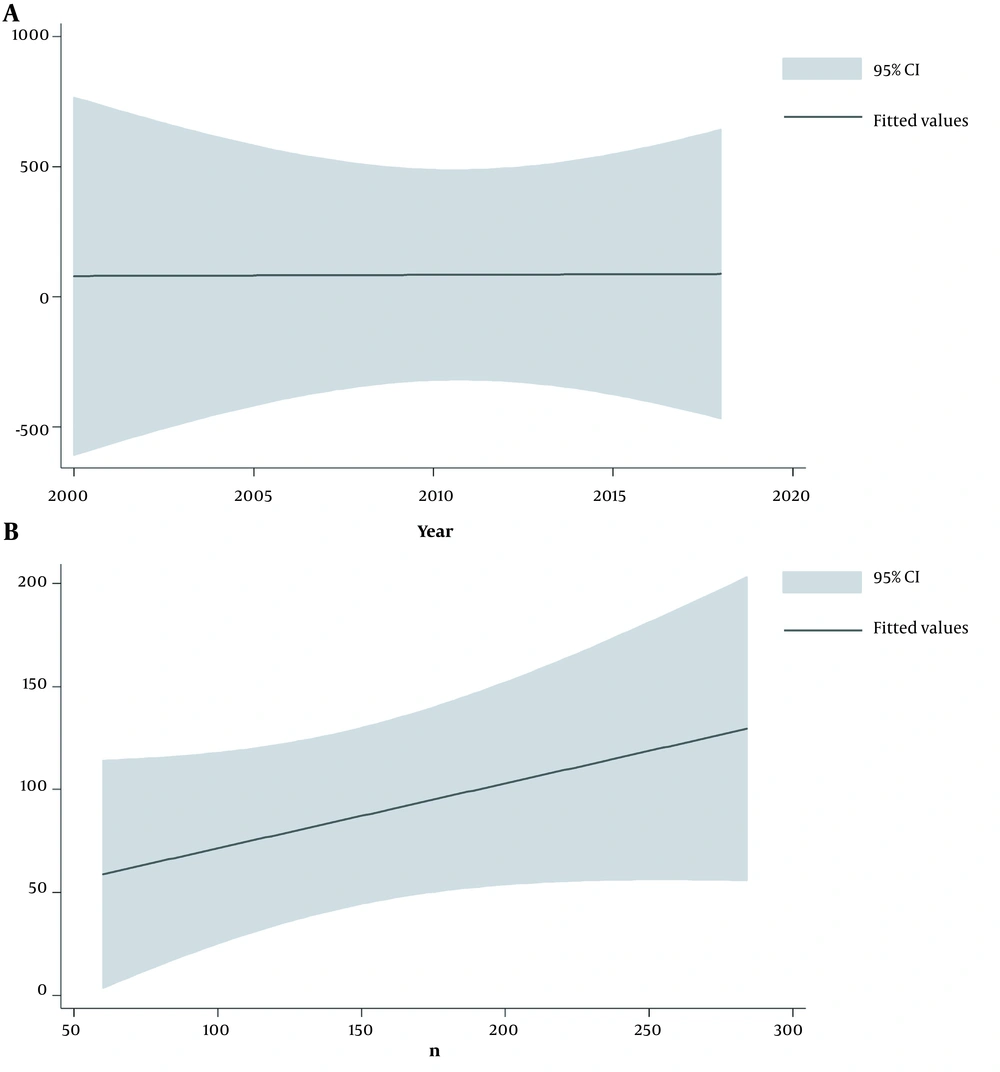
The characteristics of the reviewed studies are shown in Table 1. The results of subgroup analysis regarding the association between asthma and lead are summarized in Table 2. Figure 2 illustrates the relationship between BLL and asthma considering the studies’ years of conduction and sample sizes. The details of the studies evaluating the relationship between asthma and zinc are listed in Table 3. Figure 3 shows the meta-regression analysis of the link between asthma and zinc based on the years of studies’ conduction and sample sizes. The results based on age groups are depicted in Figure 4.
| Author | Place | Year | Total | Male | Female | Age, Mean ± SD |
|---|---|---|---|---|---|---|
| Pugh Smith and Nriagu (5) | USA | 2011 | 361 | 177 | 184 | 5.9 ± 4.37 |
| Joseph et al. (20) | USA | 2005 | 4634 | 234 | 2294 | 1.2 ± 0.5 |
| Wang et al. (21) | China | 2017 | 930 | 469 | 461 | 5.74 ± 0.77 |
| Wu et al. (22) | Taiwan | 2018 | 5866 | 3053 | 2813 | 7.5 ± 1.5 |
| Ghaffari et al. (15) | Iran | 2014 | 284 | 143 | 141 | 7.8 ± 1.2 |
| Mohamed et al. (23) | Egypt | 2018 | 60 | 32 | 28 | 44.5 ± 6.54 |
| Vural et al. (24) | Turkey | 2000 | 84 | 37 | 47 | 36.2 ± 11.7 |
| Variables | Article (n) | % | 95% CI | I2 | P-Value |
|---|---|---|---|---|---|
| Prevalence of asthma | 4 | 12 | 11 - 13 | 90.9 | 0.000 |
| Blood lead level | 4 | 2 | 2 - 3 | 98.6 | 0.000 |
| Asthma and lead | 2 | 3 | 3 - 4 | 98.7 | 0.000 |
| Blood lead level grade, μg/dL | |||||
| < 5 | 2 | 8 | 7 - 9 | 98.5 | 0.000 |
| 5 - 10 | 2 | 10 | 8 - -11 | 98.1 | 0.000 |
| > 10 | 2 | 9 | 8 - -11 | 97.2 | 0.000 |
| Age group, y | |||||
| 2 - 5 | 2 | 15 | 15 - 15 | 98.3 | 0.000 |
| 6 - 11 | 2 | 3 | 2 - 3 | 98.5 | 0.000 |
| 12 - 15 | 2 | 1 | 1 - 1 | 98.9 | 0.000 |
| Ethnicity | |||||
| American-Spanish | 3 | 30 | 26 - 34 | 97.4 | 0.000 |
| Asia-Caucasian | 3 | 33 | 29 - 37 | 97.4 | 0.000 |
| Variables and Subgroups | Article (n) | % | 95% CI | I2 | P-Value |
|---|---|---|---|---|---|
| Mean by μg/dL | |||||
| Mean zinc in the case group | 3 | 70.44 | 57.63 - 83.24 | 80.9 | 0.005 |
| Mean zinc in the control group | 3 | 74.1 | 63.86 - 84.34 | 92.6 | 0.000 |
| FEV | |||||
| Mean in the case group | 2 | 81.8 | 74.71 - 88.89 | 74.8 | 0.047 |
| Mean in the control group | 1 | 93.4 | 83.8 - 100 | 100 | 0.000 |
A, Meta-regression for investigating the relationship between asthma and blood lead level based on publication year indicating an increasing occurrence from 2005 to 2018. B, Meta-regression for investigating the relationship between asthma and blood lead level based on the sample size revealing a higher occurrence of asthma in studies with larger sample sizes.
A, Meta-regression for investigating the relationship between asthma and zinc based on the publication year indicating an increasing occurrence from 2005 to 2018. B, Meta-regression for investigating the relationship between asthma and zinc based on the sample size revealing a higher occurrence of asthma in studies with larger sample sizes
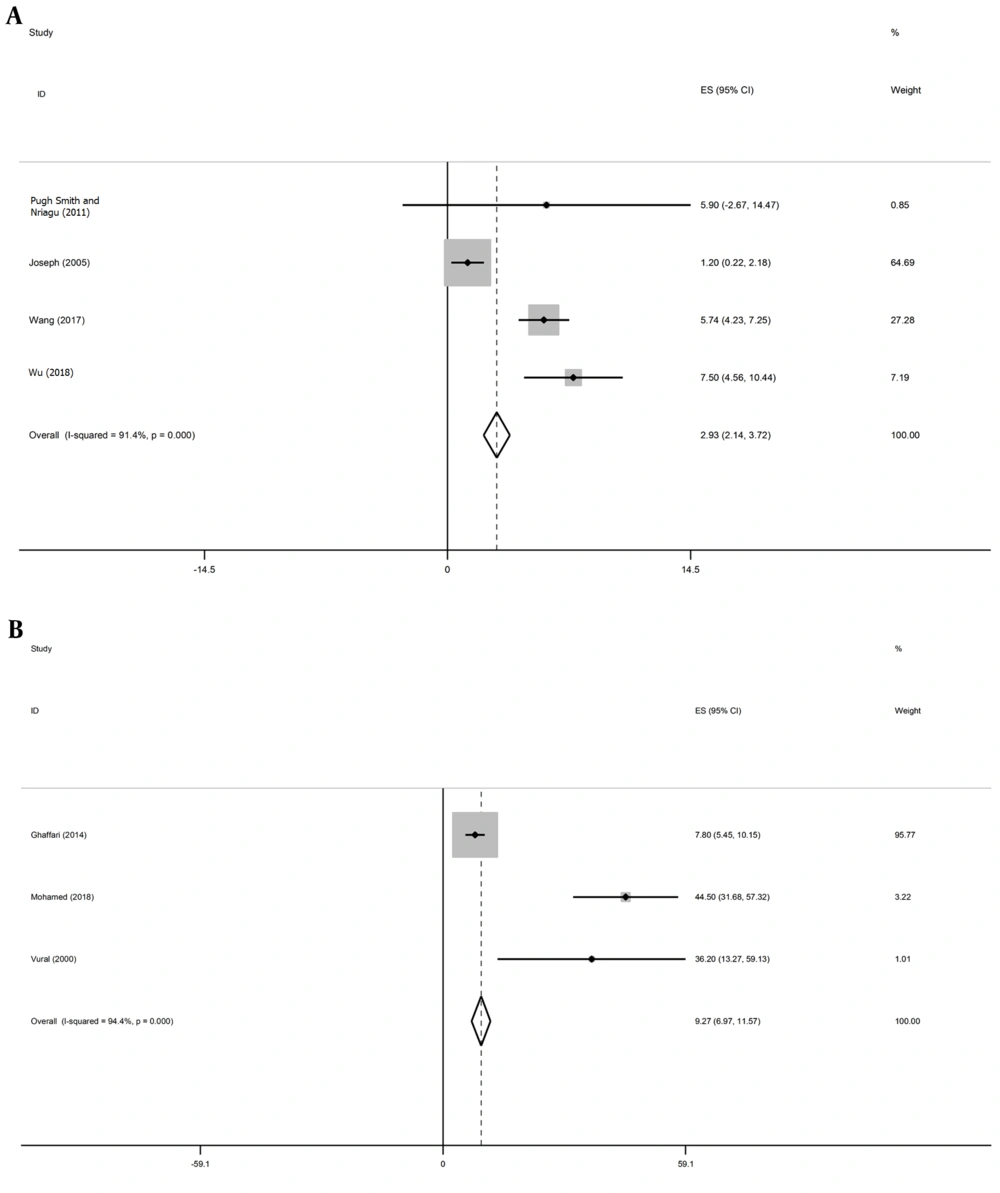
Forest plot of the mean age along with the 95% confidence interval. A, Mean Age of patients in the studies assessing the relationship between asthma and BLL was 2.93 years (95% CI: 2.14 - 3.72, I2 = 91.4%, P < 0.001). B, Mean age of patients in the studies assessing the relationship between asthma and zinc was 9.27 years (95% CI: 6.97 - 11.57, I2 = 94.4%, P < 0.001). The Middle point in each line represents the mean age in each study. The diamond shape indicates the confidence interval of the overall mean age.
5. Discussion
Asthma is one of the respiratory diseases, which has been reported to show an increasing trend in developed societies (7, 8). This systematic review and meta-analysis study aimed to assess the potential association of asthma development with lead and zinc. According to our study findings, various degrees of lead toxicity may have increased the risk of asthma, which agreed with the results from previous similar studies (20-22). Lead is a hazardous agent contributing to respiratory diseases, so it is advisable to educate people about the risks of lead exposure and inhalation in potentially polluted areas.
Increased levels of IgE and some inflammatory cytokines have been recorded in children and neonates exposed to lead during either fetal life or lactation. Also, lead targets macrophages and Th cells, which triggers the release of inflammatory mediators in vitro and in vivo. Lead exposure has been documented to associate with no change or even decreased serum immunoglobulins in laboratory animals, and there are inconsistencies about the stimulating or inhibiting effects of lead on the immune system (25).
Many researchers have asserted that lead interferes with Th cells’ maturation, deviating immune responses toward the Th2axis. Regarding the elevation of IgE level following lead exposure, it has been postulated that lead may contribute to the pathogenesis of IgE-dependent allergic diseases (25, 26).
Elevated blood lead levels can result in anemia through disturbing iron metabolism. Also, excess blood lead may accumulate in the liver, which in turn causes hepatic dysfunction. Furthermore, the infiltration of lead through the renal tissue disrupts renal function as well (20, 21). Lead poisoning is mainly treated by administrating lead chelators such as Dimercaprol and CaNa-EDTA. Since lead in patients with renal disorders is mainly excreted in bile than in urine, it is recommended that Dimercaprol should be used in this condition (21, 22).
According to our study findings, the highest ratio of lead toxicity was related to children aged 2 - 5 years. Children are particularly prone to toxicities and diseases; therefore, they should be constantly watched to protect them against such intoxications. In this regard, educating parents can play an essential role in reducing lead toxicity (21, 22).
According to the findings of this study, the occurrence of lead toxicity was not significantly different among various ethnic groups, negating a role for this factor in lead intoxication (20-22).
Zinc is an effective element positively contributing to asthma improvement, a notion that was also supported by the present review. Zinc administration can alter the FEV value, which improves asthma signs. However, contradictory results have been reported regarding the impacts of zinc on asthma symptoms and, therefore, further studies should be carried out to confirm the association of zinc and FEV with asthma. Zinc is also a vital element for growth, which highlights the great importance of avoiding zinc deficiency in children by feeding them well-balanced diets containing veal, beef, lentils, and bread. Therefore, parents should provide their children with such nutritional resources to avoid zinc deficiency in them (23, 24). In fact, parents should be informed of the essential roles of zinc in augmenting the immune system, healing wounds, preventing diarrhea, promoting skin health, and anemia prevention (23, 24).
Asthma prevalence and BLL were found to have been increasing from 2005 to 2017. This may have been attributed to air pollution and increased industrial activities during this period. It is advisable to inform people about the benefits of using masks in polluted places and preventing the inhalation of this toxic agent (20-22).
5.1. Limitations
The main limitation of this review was the non-random selection of the patients in some studies. Furthermore, the number of reported variables was low in some studies (e.g., failure of some studies to report BLL and zinc levels in individual genders).
5.2. Conclusions
In sum, lead was a dangerous heavy metal and a major risk factor for respiratory diseases, including asthma. The administration of zinc sulphate may have been beneficial in treating asthma. It was recommended that the children should be educated about appropriate safety measures in order to prevent lead poisoning and asthma among them.
5.3. Recommendations
Monitoring children vigilantly and educating them and their parents may have been effective in reducing lead toxicity and asthma in society.