1. Background
Previous studies have shown different prevalence rates for children with epilepsy who developed drug-resistant epilepsy (DRE), which was about 7 - 30% (1-4). However, it has recently been observed to be 25% (5) and requires other measures to control seizures. Among these measures, the ketogenic diet (6), vagus nerve stimulation (7), and epilepsy surgery are widely used in children with DRE and might be used in future gene therapy. To date, at least two clinical trials have shown that epilepsy surgery is more effective than anti-seizure medications in patients (mostly adults) with medication-resistant epilepsies (8, 9). Kuzniecky and Devinsky showed that epilepsy surgery could control seizures, improve quality of life, and reduce the costs of medical care (10).
To determine the epileptogenic zone in pre-surgical evaluation, multi-modal planning, such as conducting long-term video electroencephalography (EEG) monitoring (LTM), is required to describe seizure semiology and conducting high-resolution brain magnetic resonance imaging (MRI) (epilepsy protocol) (11-13). Epilepsy surgery would have the best results when the patient has structural brain pathology or lesion-induced epilepsy. In patients with normally reported MRI, the yield of epilepsy surgery is low because the epileptogenic zone could not be correctly localized. Therefore, these patients with DRE and normal brain MRI would require more sophisticated measures to determine the probable epileptogenic zone. These measures would be divided into invasive and non-invasive methods. Among non-invasive methods, positron emission tomography (PET), subtraction ictal single-photon emission computerized tomography (SPECT) co-registered to MRI (SISCOM), magnetoencephalography (MEG) (12, 14, 15), functional MRI (fMRI), and high-density EEG are the main measures. Nevertheless, these measures are expensive and, in many developing countries, not available.
Among non-invasive methods, source localization of epileptiform EEG discharges has been known as a non-expensive and practical measure to localize the probable seizure generators in the brain. This technology, in combination with high-resolution brain MRI, could be used as a reliable and valid measure in the pre-surgical evaluations of patients with medication-resistant epilepsies (16-21). In addition, in pre-surgical planning, if we decide to use invasive electrodes, source localization with solving EEG inverse problem can be used to limit the predicted area of the epileptogenic zone and finally lead to reduced cost of pre-surgical evaluations by decreasing the number of invasive electrodes and minimal injury to brain tissue (22). Source localization could be applied using standard digital EEG devices, which have been available in many developing countries.
To the best of our knowledge, no study has been conducted specifically in Iran a developing country, to show the application of source localization in the pre-surgical evaluation of children with DRE. The present study adopted a new method for obtaining general results. To this end, this study used 3T MRI data extraction individual head model (23), processing interictal signals that have already been captured in the LTM EEG laboratory, using the known source localization method brain electrical source analysis (BESA), and cortical classical LORETA analysis recursively applied (CLARA) to help epileptologists in determining the probable seizure generators in children with DRE, especially in those with normal brain MRI. In this method, several steps were taken to achieve the aims. First, the LTM and MRI data were extracted from seven patients. Then, interictal epileptiform discharges (IEDs) were detected and clustered. In the next step, principal component analysis (PCA) was used for dimension reduction, and propagation was decided on.
2. Objectives
Afterward, this study utilized BESA and cortical CLARA methods for source localization and, in the next stage, co-registering obtained sources using individual and age template head models. Finally, the results of the proposed methodology were reviewed and compared to other clinical interpretations.
3. Methods
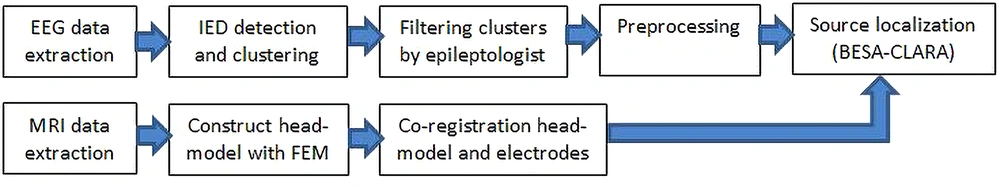
This study aimed to solve the interictal EEG inverse problem with a non-invasive approach to detect the seizure onset zone (SOZ). In this regard, this study took several processing steps on EEG signals and prepared them for implementation in the source localization procedure with the BESA software (version 7). The proposed methodology is shown in the block diagram framework (Figure 1).
3.1. Inclusion Criteria of Patients
In this study, seven patients (mean age: 11.4 years) were involved (Table 1).
| Patient ID | Age (y) | Starting Seizureage | Primary MRI Report | Clusters | IED (%) | Method 1 | Method 2 | Method 3 | Propagation |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 16 | 12 years | None | 1 | 75 | Right | Left frontal | Right temporal | No |
| 2 | 25 | Right | Right frontal | Right temporal | No | ||||
| 2 | 16 | 15 years | Suspicious lesion in left temporal | 1 | 100 | Left | Left frontal | Right temporal | No |
| 3 | 4 | 2 months | None | 1 | 76 | Left | Left frontal | Right sublobar | No |
| 2 | 24 | Left | Left frontal | Right parietal | No | ||||
| 4 | 7 | 2 years | None | 1 | 63 | Left | Left frontal | Left frontal | Yes |
| 2 | 36 | Right | Left temporal | Right sublobar | No | ||||
| 3 | 1 | Right | Right parietal | Left temporal | No | ||||
| 5 | 17 | 1 year | None | 1 | 41 | Right | Left frontal | Right frontal | No |
| 2 | 34 | Right | Right frontal | Left parietal | No | ||||
| 3 | 25 | Left | Left frontal | Left frontal | No | ||||
| 6 | 8 | 6 months | Right mesial temporal sclerosis | 1 | 97 | Left | Left frontal | Right occipital | No |
| 2 | 2 | Left | Left frontal | Right temporal | Yes | ||||
| 3 | 1 | Left | Right temporal | Right temporal | No | ||||
| 7 | 12 | 7 years | None | 1 | 43 | Left | Left frontal | Left frontal | No |
| 2 | 41 | Right | Right frontal | Right frontal | No | ||||
| 3 | 16 | Right | Right parietal | Left temporal | No |
Prediction of Seizure Onset Zone with the Proposed Methodology Using Method 1 (BESA with Age Template Head Model), Method 2 (Cortical CLARA with Individual Head Model), and Method 3 (BESA with Individual Head Model)
This study included all those patients with focal epilepsy who were medication-resistant according to the International League Against Epilepsy definition (24). Therefore, both MRI-negative and positive patients were in the inclusion criteria of this study. All of these patients had been referred from the comprehensive epilepsy program of the Children’s Medical Center, Tehran, Iran.
3.2. Patients Population and Source of EEG
The LTM data were recorded with 19 scalp electrodes for a minimum duration of 24 hours at the Children’s Medical Center Hospital. This center is a university hospital affiliated with Tehran University of Medical Sciences and acts as a tertiary referral center for all those children with DRE who are referred from all over the country. All the children underwent LTM. All the LTMs were conducted in a room with two dedicated beds using Neurofax EEG-1200 diagnostic and monitoring platform 64 channels (Nihon Kohden Singapore Pte Ltd., 1 Maritime Square, # 10-34 HarbourFront Centre, Singapore 099253) with dedicated cameras that had the capability of night vision.
All video-EEG monitoring was conducted using silver-silver chloride cup electrodes. The electrodes were applied using collodion and conductive gels provided by the Nihon Kohden brand. The electrodes were placed according to the 10 - 20 international system based on the American Clinical Neurophysiology Society guidelines. Moreover, additional electrodes, such as T1 and T2, were used as indicated. In every recording, at least two electromyography (EMG) electrodes, electrocardiography (EKG), and extraocular movement electrodes were also utilized. During all recordings, the sound was recorded using an omnidirectional microphone that was installed on the ceiling of the room. The sampling rate during all recordings was 1000 per second. The recording sensitivity was set at 7 µv/mm. The low- and high-frequency filters were set at 0.5 and 70 Hz and changed if needed during the review. All the recordings were reviewed using appropriate typical average and bipolar montages. The Local Committee on Human Research of Tehran University of Medical Sciences approved the ethics of this study (IR.TUMS.MEDICINE.REC.1398.176).
3.3. Imaging
Conventional MRI was performed with 3 tesla general electric medical system scanners using a 24-channel head coil. The MRI protocols for mapping the seizure patients were provided by Neuroimaging and Analysis Group (NIAG) of Tehran University of Medical Sciences, which contains the following sequences:
- Sagittal three-dimensional volumetric T1 fast spoiled gradient recalled [repetition time (TR): 8.5 ms, time to echo (TE): 3.2 ms, the field of view (FOV): 256 mm, acquisition matrix: 256 × 256, voxel size: 1 × 1 × 1 mm3].
- Sagittal cube T2 (TR: 3000 ms, TE: 106.4 ms, FOV: 256 mm, acquisition matrix: 256 × 256, voxel size: 1 × 1 × 1 mm3).
- Coronal cube T2 fluid attenuation inversion recovery (FLAIR) (TR: 6000 ms, TE: 112.9 ms, FOV: 224 mm, acquisition matrix: 512 × 512, voxel size: 0.4 × 0.4 × 1 mm3).
- Sagittal double inversion recovery (TR: 7000 ms, TE: 90.7 ms, FOV: 256 mm, acquisition matrix: 512 × 512, voxel size: 0.5 × 0.5 × 1 mm3).
- Coronal T1 FLAIR (TR: 3410 ms, TE: 39.3 ms, FOV: 224 mm, acquisition matrix: 512 × 512, voxel size: 0.4 × 0.4 × 4 mm3).
- Axial susceptibly weighted imaging (TR: 54.5 ms, TE: 23.6 ms, FOV: 224 mm, acquisition matrix: 512 × 512, voxel size: 0.4 × 0.4 × 1 mm3).
For registration and brain model extraction, the volumetric T1 and T2 images were used. Regarding this issue, different head tissue components were considered (especially white and grey matter, cerebrospinal fluid, skull bone, and skin) to generate a model that clarifies how neural electric currents produce an electric field and differences in electrical potentials at external electrodes. This method that uses EEG from electric current dipoles and leads to achieving a model outside the head is known as a forward problem or solving EEG forward problem. Several methods, such as the boundary element method, finite element method (FEM), and finite difference method, were used to solve the forward problem of EEG source analysis. In this study, the FEM method was used (23, 25).
3.4. IED Detection
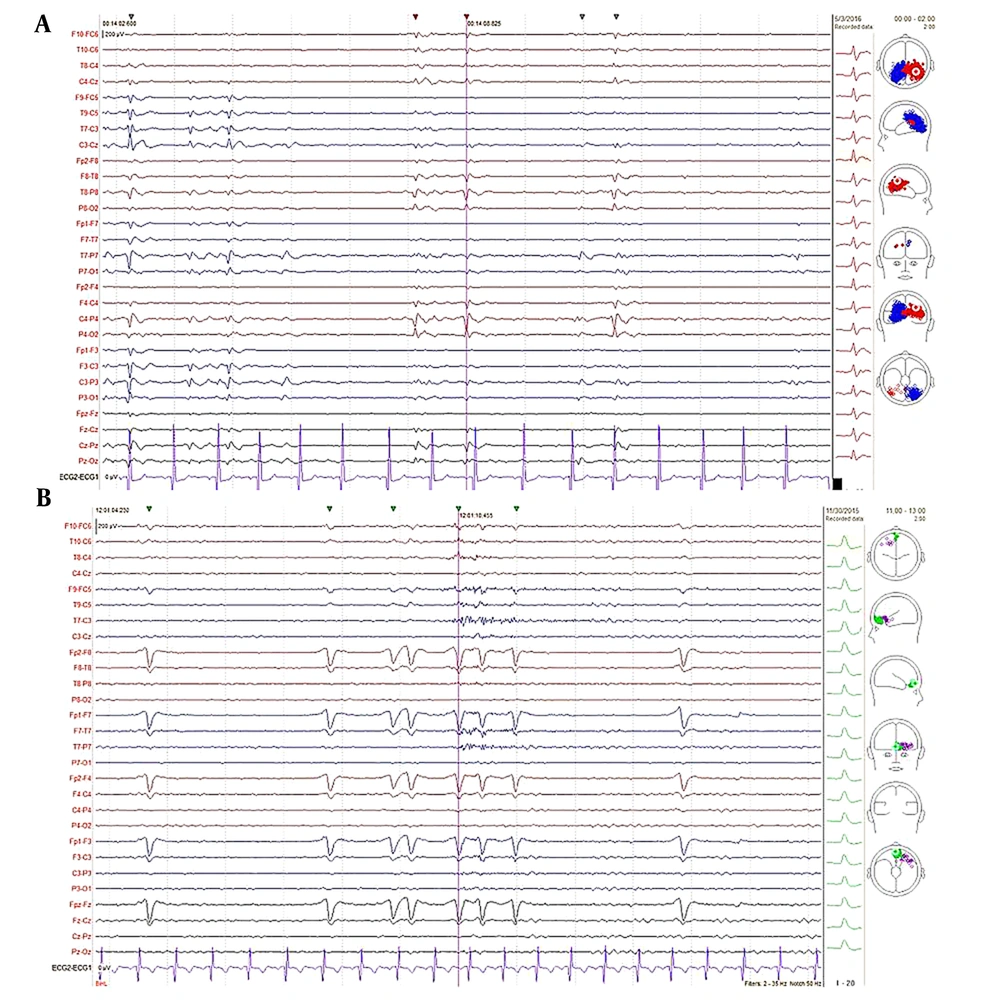
Epileptiform discharges are divided into interictal and ictal. Interictal signals have fewer artifacts than ictal signals. Most importantly, for the EEG inverse problem, it is necessary to use EEG signals with the lowest possible artifacts. Moreover, using pathologic samples after epilepsy surgery, it has been shown that interictal spikes have 84% accuracy in source localization (16). The IEDs have several morphology types, such as spikes, sharp waves, poly spikes, poly spikes with wave, and spike with wave; however, the most valuable morphologies of IEDs for source localization are spikes and sharp waves since these two groups can be produced by a limited area in the brain and were mainly interested in extracting the SOZ in pre-surgical evaluation (26). To extract these transients, one of the popular and efficient methods was used for the fast, automatic detection of IEDs and clustering (27). Then, these clusters were filtered by expert neurophysiologists to detect correct spikes and sharp IEDs, removing artifacts and unreliable signals. An epileptologist reviewed several members of each cluster in different electrode montages in this step to determine cluster membership (Figure 2).
Assessment clusters by an epileptologist; A, sample interictal epileptiform discharge (IED) pattern of one cluster automatically detected and confirmed by an epileptologist; B, sample IED pattern of one cluster automatically detected but rejected by Epileptologist due to being the signal of eye blink artifact. Brain electrical source analysis epilepsy software 2 is used for automatic IED detection
3.5. Preprocessing
The EEG signal can be contaminated by several artifacts, such as EMG, electrooculography (EOG), EKG, and background EEG. To decrease the impact of these artifacts and obtain a clean IED, preprocessing should be used. The preprocessing methods can be grouped into three categories: time domain, frequency domain, and time-frequency domain. The IEDs usually have a frequency band of 2 - 35 Hz and a duration of 20 - 200 ms, and the amplitude of their signals is mostly lower than 250 µv (28). Then, for increasing signal-noise ratio, this study processed averaging between the IED patterns of each cluster to remove other backgrounds EEG and bandpass filter (5 - 45 Hz) and final segment from 250 ms before to 150 ms after the peak of IED patterns for source localization procedure. Previously has been assessed that the background noise in IEDs produces scattering in the localization of principal sources (29).
3.6. EEG Source Localization
To answer the question, “where is the source of signals in the brain using scalp electrodes?” we would encounter an EEG inverse problem. The inverse problem is an ill-posed problem because it is estimated that there are 86 billion neurons (sources) in humans’ brains (30); however, the number of sensors in the best situation possible (high-density EEG) is 256 electrodes. For solving the EEG inverse problem, there are two main solutions, including discrete source methods (ex: Cortical CLARA) (31), and distributed source methods. The application of discrete source methods, such as the BESA method (32), to localize focal IEDs has been shown in previous studies (33). In the current study, the BESA and cortical CLARA were used to achieve generalization and the most robust results for EEG source localization. Additionally, for better lateralization of SOZ, this study incorporated BESA with the age template head model alongside two other methods (BESA and cortical CLARA with an individual head model).
This study used PCA on average IED clusters in the source localization procedure for two reasons: firstly, to detect maximum dominated activity in an IED and explain it using dipole fitting source localization (34), and secondly, to detect propagation in the onset-peak interval of IED source localization (35). The PCA method steps are as follows (36): (1) Taking the mean of the data and subtracting it from the primary data; (2) calculating the covariance matrix; (3) extracting the eigenvalue and the eigenvector of the covariance matrix; (4) constructing data with each principal component separately and rating them according to their coverage of variation in data.
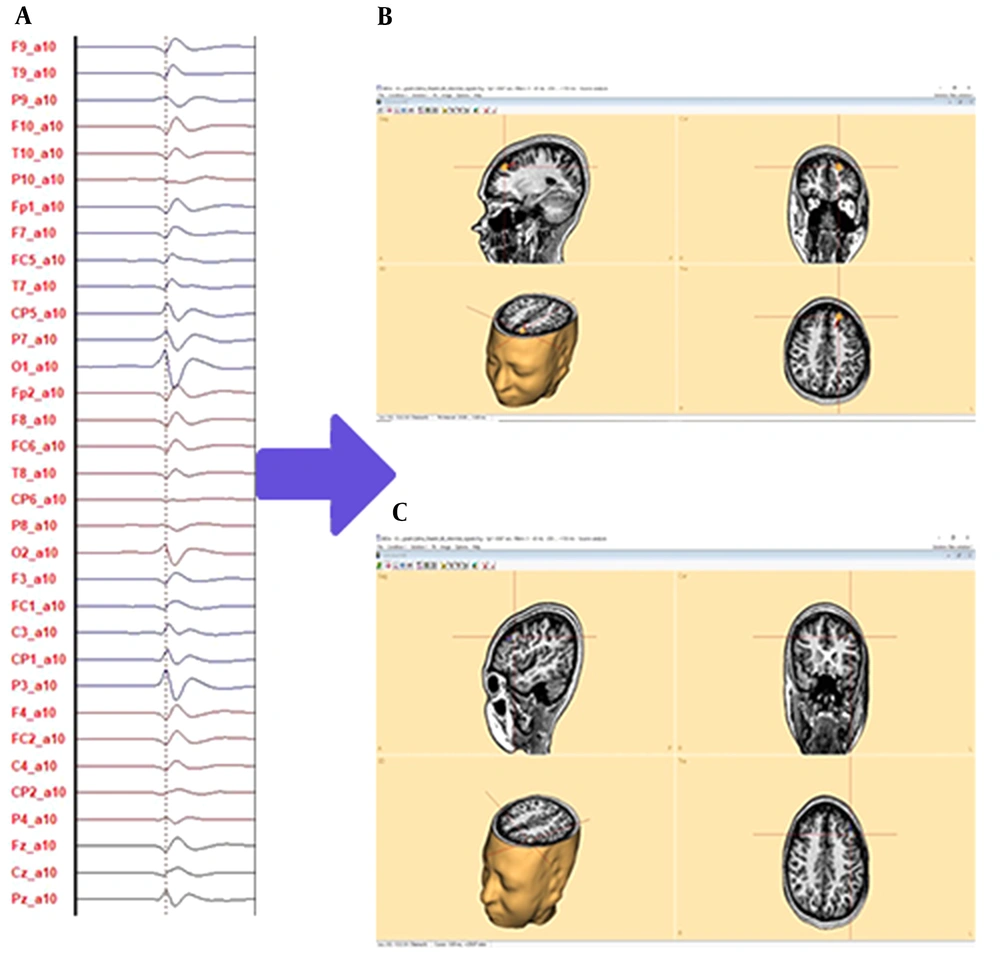
For detecting propagation, it is possible to check the variance of the second principal component; if it is above 5%, propagation has occurred, and otherwise, propagation does not occur. Once propagation has occurred, the BESA method employs a two-regional dipole, one for the onset source and one for the peak source (from 20 ms before peak-to-peak point location), and the onset source is retained while the peak source is ignored. For the cortical CLARA method, the time point of maximum amplitude in the onset source is used for source localization. When propagation does not occur, the peak source in the BESA method and the peak time in the cortical CLARA method have been used for IED pattern source localization (35). Figure 3 depicts the predicted SOZ of the case that has non-lesional focal epilepsy using the proposed methodology that has been introduced in this study.
Sample interictal epileptiform discharge (IED) source localization; A, IEDs without propagation; B, prediction of seizure onset zone (SOZ) of IED by brain electrical source analysis method source localization with proposed methodology; C, prediction SOZ of IED by cortical classical LORETA analysis recursively applied method source localization with the proposed methodology in three types of plane: sagittal, coronal, transverse, and three-dimensional view. Both methods predict that the SOZ is on the left frontal lobe.
4. Results
Seven patients (age range 4 - 17 years) were studied during this study. In each patient, it was tried to lateralize and localize the SOZ according to the clusters of epileptiform activity (Table 1).
Then, these results were compared to other diagnostic modalities, such as MRI and epileptologist diagnosis (according to semiology, the onset of ictal epileptiform discharge, and IEDs) (Table 2).
| Patient ID | EEG (Ictal Onset) | EEG (Inter-Ictal) | EEG | MRI | Clusters LOC (%) | PM (LAT) | PM (LOC) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | None | RFC | RF | None | RT (75) | RFT (25) | R | RFT | ||
| 2 | None | LF | Need more events | LT | (100) LF | L | LF | |||
| 3 | LT | None | LT | None | LF (76) | LF (24) | L | LF | ||
| 4 | LT | LT | LT | RP | LF (63) | LT (36) | RP (1) | L | LFT | |
| 5 | Left | Left | Left | None | RF (41) | RF (34) | LF (25) | R | RF | |
| 6 | LFC | LCP | Need more events | RT | LF (97) | LF (2) | RT (1) | L | LF | |
| 7 | RF | LF | LF | Need more events | None | LF (43) | RF (41) | RP (16) | R | RFP |
Comparing Results of the Proposed Methodology to Other Modalities Regarding Seizure Onset Zone Location
The number of IED clusters found for each patient was between 1 and 3 clusters. Based on the present proposed methodology, every IED cluster comprises several IED signals, varying from 1 to 6567. To fuse the result of cluster sources for SOZ lateralization, as mentioned before, this study incorporated BESA with the age template head model alongside other two methods. Firstly, this study used the majority vote method to lateralize each cluster’s SOZ and localize it by source regions on the chosen hemisphere. Secondly, this study lateralized the SOZ of each patient based on the frequency (number) of IEDs in the right and left hemispheres. Thirdly, this study localized the SOZ based on existing regions in the selected hemisphere. In this procedure, subcortical sources were ignored since most SOZs (more than 80%) occur in the brain cortex.
For example, in ID number 4, there are three clusters, including cluster number 1, which is localized on the left frontal (LF), cluster number 2, which is localized on the left temporal (LT), and cluster number 3, which is localized on the: Right parietal (RP). Since 99% of IEDs are produced on the left side, this study lateralized the SOZ on the left hemisphere; therefore, the SOZ was located in the left frontotemporal region.
According to the diagnosis of the epileptologist, the MRI results, and the results of this study (Table 2), some remarks could be made.
- Remark 1: This study showed 71% (5 of 7) lateralization accuracy.
- Remark 2: The location of the SOZ and containing lobe in 50% (2 of 4) patients was similar to the LTM report. Moreover, the present defined SOZ was localized on a precise region of the lobe; therefore, these data might be good for invasive EEG monitoring.
- Remark 3: In two patients, the region of SOZ did not match the LTM report; however, in one of them, the source for the cluster adapted to the LTM result.
- Remark 4: Aside from patient three, the epileptologist did not notice an IED by visual revising the LTM. This study detected two IED clusters.
- Remark 5: In two cases, the propagation occurred on cluster sources.
- Remark 6: In a patient with ID number 4, the primary MRI report did not show any epileptic abnormality; however, after obtaining the findings, the images were re-checked by the radiologist, and he found an abnormality on the RP.
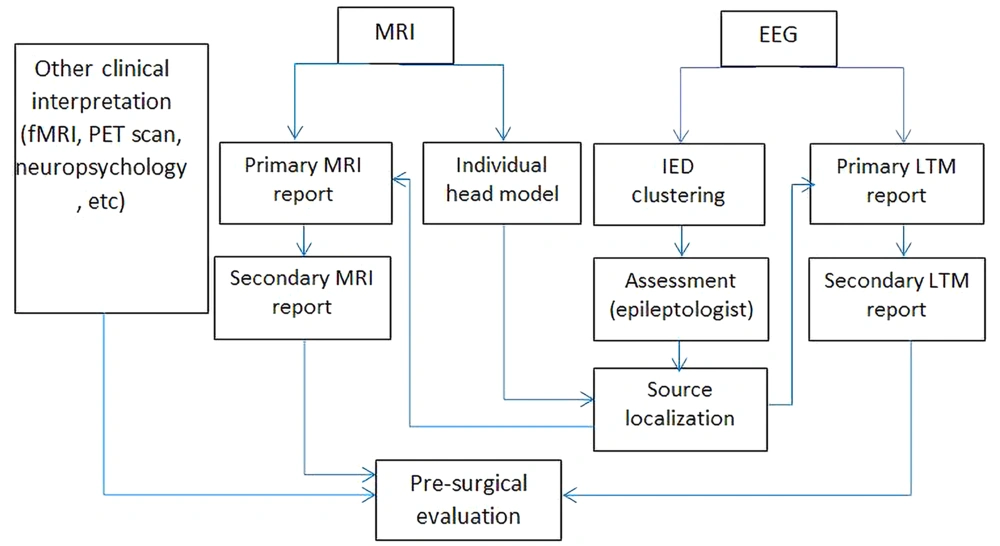
Based on the present experience and the remarks mentioned above, this study investigated a specific framework for using EEG source localization with BESA software to improve other clinical interpretation methods in pre-surgical evaluation (Figure 4).
5. Discussion
In most of this study’s cases (71%), the proposed methodology was in accordance with the LTM results. This could increase the value of SOZ lateralization. In addition, this study observed that in some cases (about 50%), EEG source localization increases the information about the possible SOZ regions in the predicted hemisphere. For example, in ID-3, it is predicted that the LF will be the source of the seizure. This finding adds more information and changes the pre-surgical evaluation for using invasive electrodes in both the temporal and frontal regions of the left hemisphere.
The current study showed that the output of electrical source imaging (ESI) could help refine the imaging findings. In one case, the radiologist confirmed the presence of heterotopic gray matter on the RP lobe after revising MRI. This pathologic region is in correlation with a predefined SOZ using the present study’s methodology. Heterotopia is a condition characterized by the incomplete migration of neurons from near the ventricles to the cortical regions, thereby resulting in neuronal complexes and activation in abnormal locations that could be seizure-inducing regions. Therefore, when focal epilepsy and seizures are observed that are stereotypic even on primary non-lesional MRI, it is necessary to look for a lesion on the brain. As a result, using source localization in addition to LTM is an appropriate factor for precise SOZ mapping, particularly in cases with normal-appearing MRI.
In two patients where the epileptologist could not come to a firm conclusion, the present study’s method came into play and added to the existing data; therefore, a final decision could have been made. Although in this issue, this study used low-density EEG, based on Foged et al. study (11), there were no significant differences in comparison to high-density EEG findings. The evaluation using this available equipment in a developing country could be helpful and alter the patient’s fate in advance. Therefore, the present study constructed a framework with regard to using EEG source localization for pre-surgical evaluation in Iran (Figure 4). Surgery and removing the SOZ are proper standard procedures for children with DRE focal epilepsy, and if this procedure is performed at an earlier age, the children would be able to experience normal development. Poor diagnostic methods and late decisions for such patients have resulted in irreversible damage to the brain, and these children are born with an abnormal cognitive network that affects their social interaction, intelligence quotient, and cognition.
One of the limitations of LTM is that it cannot include all the occurring events; previous studies have shown that 15 - 27% of cases underwent LTM without an event. Therefore, it is required to conduct further LTM to capture seizure events in the patient. According to this issue, the present study’s methodology for working with IEDs does not require any seizures; consequently, the duration of LTM could be reduced, and as a result, the cost of treatment would be much lower than before (37-41).
The efficiency of source localization with EEG (11) and MEG (42) has been previously proven; however, MEG (about 2,000,000 USD), high-density EEG (about 25,000 USD and more), fMRI (about 225,000 - 500,000 USD and more), and PET (about 225,000 - 750,000 USD) devices are expensive (43-47). Nevertheless, source localization with the present study’s methodology can be applied using standard digital EEG devices (about 1,000 - 25,000 USD) (48) that have been available in many developing countries. This study is the first step, and there are some limitations, such as the number of patients, lesional patients, epilepsy surgery, and the following outcome for comparing the present study’s methodology to other studies. Therefore, we would like to perform further studies to examine more patients with different features and provide specific room for epilepsy surgery in children to improve the present study’s methodology in the future.
In this study, it was tried to compare the results of ESI to other non-invasive pre-surgical methods, and intracranial EEG results were not incorporated. Therefore, one of the limitations was not using invasive electrodes as the better gold standard than other methods (e.g., MRI and LTM). It is suggested to address this issue in future studies.
5.1. Conclusions
This study used ESI with a specific methodology for focal epileptic children with DRE. The novelty of the current study’s proposed methodology can be summarized as the usefulness for cases with insufficient data in other modalities, such as patients whose MRIs were negative, for patients with no ictal information and low events, in situations where we miss some interictal signals in visual reviewing of LTM data, and for predicting the region of SOZ with three ESI methods rather than one (11). According to limitations in a developing country, multi-modal studies (i.e., 3T MRI, LTM, and ESI) with a specific valuable protocol are not feasible. This investigation defined a pilot study with a 7 data sample for the first step. These findings, based on the small sample size, suggest that ESI based on combining ensemble methods improves information for children with focal DRE. It is hoped that future studies with large sample sizes show the role of ESI in developing countries more than before.
.jpg)