1. Background
Hypoxic-ischemic encephalopathy (HIE) is a brain injury caused by oxygen deprivation or asphyxia, observed in about 1 - 3% of term or near-term neonates during the prenatal period, leading to a mortality rate of about 20% and accounting for 22% of the neonatal deaths worldwide (1). With an annual incidence of more than 12 000 infants in the USA, perinatal HIE occurs in 1 to 3 cases per 1000 term births (2). It is considered the second and third leading cause of neonatal and childhood deaths, respectively (3). HIE is a progressive process that causes remarkable neuronal cell death within hours to days after the initial attack and can result in permanent damage to the central nervous system (CNS) tissues. Brain injury, considered one of the major problems in the survived newborns (4), leads to persistent neurological complications, such as learning difficulties, attention deficit disorder, cerebral palsy, epilepsy, visual disorders, and severe cognitive and developmental disorders (5), imposing high economic costs on the healthcare system and society (6).
After resuscitating the neonate from asphyxia, a secondary energy defect begins after a latent phase lasting 6 - 15 hours. This stage, characterized by seizures, cytotoxic edema, the release of excitotoxins, and impaired oxidative metabolism in the brain (followed by neuronal cell death) (3), corresponds to the treatment window that can help reduce the brain damage (7). It is suggested to predict the asphyxia severity by abnormal electroencephalography (EEG) and perform advanced neonatal resuscitation to reduce death (8). Hypothermia, also known as targeted temperature management (TTM) and initiated in the latent phase (within the first 3 hours after asphyxia), is suggested as an effective neuroprotective therapy that can lead to improved outcomes (9, 10). However, more than 40% of the neonates who receive hypothermia die or suffer from moderate to severe disabilities, including cerebral palsy, intellectual impairment, and epilepsy (9, 11). Therefore, adjuvant neuroprotective therapies are required.
Erythropoietin (EPO) is a hematopoietic hormone with multiple functions involved in neurogenesis, angiogenesis, and immune response, which also acts as a cytokine with anti-apoptotic activity (12). Its synthetic form, approved by the US Food and Drug Administration (FDA), is a commercially available and easy to administer agent with minimal side effects (13) and has been proven to have remarkable neuroprotective and neuro-regenerative effects in ischemic brain injuries (14-16). It has also been suggested as an effective adjunctive therapy to be used along with TTM in neonates with HIE (17-19).
2. Objectives
Nevertheless, the previous studies have several limitations, such as limited sample size, lack of a matched control group, and varied doses and administration methods used. Therefore, in the present study, we aimed to determine the effect of the addition of EPO to the standard treatment (head cooling) on the short-term outcomes of neonates with HIE.
3. Methods
This clinical trial was conducted on newborns with HIE hospitalized in Fatemieh Hospital, affiliated with Hamadan University of Medical Sciences, Hamadan, Iran, from 2019 to 2020. The study sample size was calculated at 18 patients in each group with an allocation ratio of 1:1, considering the effect size at 0.9, α error at 0.05, and study power at 0.8; the sample size of the control group was considered at 44 neonates to increase the test power. All neonates who had the above-mentioned indications received head cooling in both groups. Full-term neonates with gestational age (GA) ≥ 36 weeks, birth weight (BW) ≥ 1800 grams, moderate to severe HIE (stages II and III) according to the Sarnat scoring tool (20) (with at least 1 criterion out of 6 diagnostic criteria of HIE), were included in this study. Also, infants with congenital anomalies, such as anorectal malformations, genetic syndrome, microcephaly, intracranial hemorrhage from head trauma or skull fracture, or bleeding disorders, were excluded.
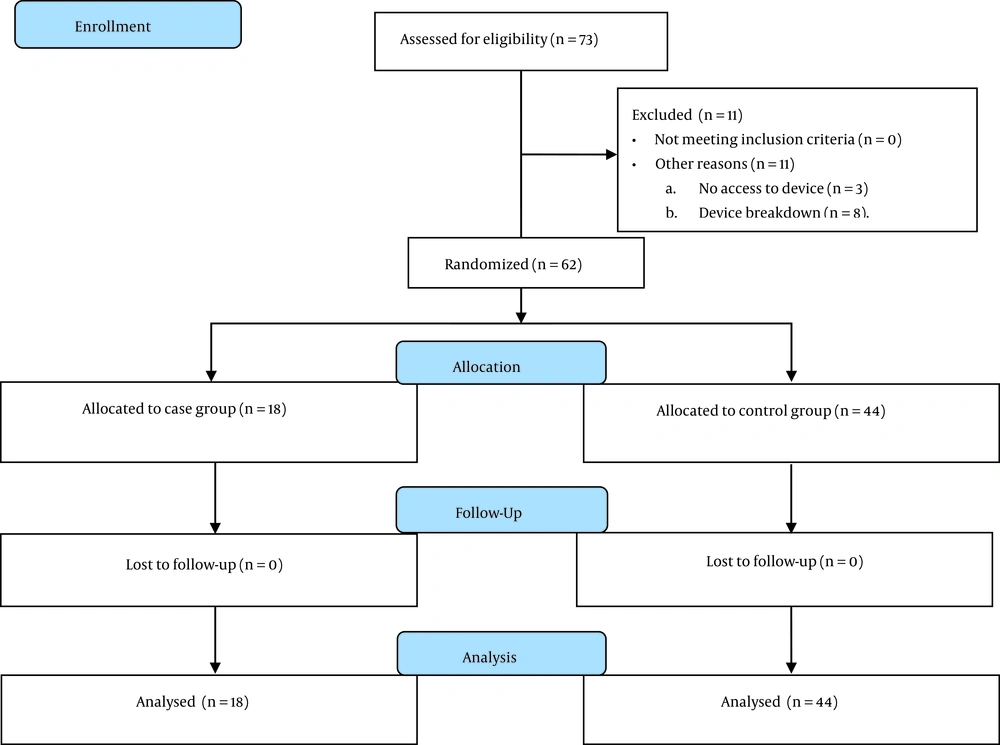
All neonates who had the inclusion criteria were included in the intervention group based on the census method; only 1 neonate who had the inclusion criteria was not included in the study, which was because of the fact that 2 neonates were referred simultaneously, and we only had 1 Cool Cap device; therefore, the neonate who did not receive cooling was not included for the intervention group to receive EPO. Also, in the control group, all neonates indicated for cooling were included in the study; during the considered period for inclusion of the control group, 10 neonates did not receive head cooling, although they met the clinical indications because of the simultaneous referral of neonates and access to 1 device (N = 2) or the temporary device breakdown (N = 8). The eligible neonates (N = 62) were enrolled in the study using the available sampling method for 15 months (Figure 1).
The intervention group received hypothermia plus 1000 IU/kg/d/IV EPO (CinnaGen, Iran) on the first 3 days and then every other day on days 5, 7, and 9 (a total of 6 dosages). The neonates who received hypothermia during the previous years at this center were considered the control group. Hypothermia began within 6 hours of birth and continued for 72 hours. The temperature was monitored by an abdominal skin probe, and the rectal temperature was maintained between 34 - 35°C. Hypothermia was performed using a Cool Cap device (Olympic Medical Cool Care System, Olympic Medical, Seattle, WA, USA) for all neonates diagnosed with HIE who had the following inclusion criteria:
Group A criteria: GA ≥ 36 weeks, 10-minute Apgar score ≤ 5, continuous need for resuscitation (ventilation by mask or tracheal tube), acidosis (pH of < 7 in the arterial blood sample of the umbilical cord), base deficit ≥ 16 mmol in the blood sample of the umbilical cord, venous or arterial sample, 1 hour after birth.
Group B criteria: The neonates with moderate to severe encephalopathy with a decreased level of consciousness with at least 1 of the following conditions: hypotonia, abnormal reflexes, weak or no sucking, and clinical seizure.
If the patient is paralyzed, we considered group B criteria as abnormal and considered seizure and/or moderate to severe abnormality (score II or III) in at least 1 amplitude-integrated EEG (a-EEG), taken until 1 hour after delivery with an interval of more than 30 minutes after administration of venous drugs.
When it was necessary, anticonvulsant therapy was administered. The study was not blinded, and the nurse who gave the interventions to the neonates and the researcher who recorded and analyzed the data were aware of the group allocations.
A researcher-made checklist was utilized to collect the study’s information, including GA, neonatal weight, appearance, pulse, grimace, activity, and respiration (Apgar) score of 1 and 5 minutes, HIE severity, need for mechanical ventilation, resuscitation, seizure, single/multiple anticonvulsant medications, length of stay (LOS), oral feeding initiation and its duration, and duration of consciousness. Thrombocytopenia was considered as platelet count < 150 000/mm3, checked in complete blood count (CBC) twice weekly in the serum sample of the neonate, and bradycardia as heart rate < 100/min (measured by sustained cardiorespiratory monitoring). Cutaneous necrosis was diagnosed by inspection.
In the intervention group, a-EEG was performed using the Olympic CFM 6000 device. For this purpose, neonates’ skin was massaged at the sites of lead connections: the black electrode at the center of the neonates’ forehead and as close to the hairline as possible, and the other 2 (yellow and red) were 3.7 cm to the right and left of the first site. The results were recorded as moderate, severe, and severe abnormal changes with seizures. For the control group, the results of a-EEG were incompletely recorded and thus could not be used.
Brain magnetic resonance imaging (MRI) was performed using a Siemens device (Germany) by the technician and reported by a radiologist. MRI was performed in 16 neonates in the intervention group (not performed in the other 2 because of unstable conditions/expiration), as well as in 13 neonates of the control group (the rest did not give consent or were discharged before completing the 72-hour treatment). The primary outcomes of the study included the in-hospital mortality of the neonate, occurrence of a seizure, LOS, initiation of oral nutrition, and duration of consciousness; the secondary outcomes included the side effects of the treatments, such as thrombocytopenia, bradycardia, and cutaneous necrosis.
3.1. Statistical Analysis
The data were analyzed using SPSS version 22 (SPSS Inc, Chicago, Ill, USA). Chi-square and Fisher’s exact tests were used to compare nominal and qualitative variables, as well as the student t-test to compare quantitative variables, between the 2 groups. P values less than 0.05 were considered statistically significant.
4. Results
A total of 62 neonates were enrolled (18 in the EPO group and 44 in the control group). Most of the neonates were male in both groups (61% in the EPO and 56% in the control group; P = 0.75). Table 1 summarizes the results of comparing the mean BW, GA, Apgar scores (1 and 5 minutes), LOS, and the onset of oral feeding between the intervention and control groups, indicating no significant difference between the groups (P ≥ 0.05; Table 1). The mean duration of consciousness was 6.92 ± 5.7 and 7.25 ± 4.53 days in the intervention and control groups, respectively (P = 0.629).
| Variables | Erythropoietin (EPO) Group | Control Group | P Value a |
|---|---|---|---|
| Gestational age (weeks) | 38.44 ± 1.46 | 38.63 ± 1.63 | 0.44 |
| Birth weight (g) | 3023.33 ± 466.17 | 3128.63 ± 495.85 | 0.67 |
| Apgar score (1 min) | 2.69 ± 1.66 | 2.11 ± 1.42 | 0.19 |
| Apgar score (5 min) | 5.06 ± 1.88 | 4.06 ± 1.63 | 0.05 |
| Hospital stay (day) | 21.22 ± 9.69 | 21.54 ± 12.36 | 0.92 |
| The onset of oral feeding (days) | 7.25 ± 4.54 | 6.96 ± 5.01 | 0.67 |
a The results of the student t-test; P values less than 0.05 were considered statistically significant.
Table 2 shows the results of comparing the frequency of other clinical conditions between the 2 study groups. As shown, the frequency of disease stages, single/multiple anti-seizure medications, resuscitation, seizure, mechanical ventilation, thrombocytopenia, bradycardia, and cutaneous necrosis were not significantly different between the two groups. (P > 0.05), but the frequency of neonates who initiated oral nutrition was higher in the control group than in the intervention group (P = 0.04).
| Erythropoietin (EPO) Group | Control Group | P Value b | |
|---|---|---|---|
| Disease stage | 0.22 c | ||
| II | 10 (55.6) | 17 (38.6) | |
| III | 8 (44.4) | 27 (61.4) | |
| Drug history | 0.66 c | ||
| Single drug therapy | 12 (64.7) | 22 (52.4) | |
| Multiple drug therapy | 6 (35.3) | 20 (47.6) | |
| Neonatal resuscitation | 17 (94.4) | 43 (97.7) | 0.53 d |
| Oral feeding initiated | 16 (88.9) | 28 (63.6) | 0.04 d |
| Seizure | 17 (94.4) | 42 (95.5) | 0.29 d |
| Use of mechanical ventilation | 16 (88.9) | 37 (84.1) | 1.00 d |
| Thrombocytopenia | 5 (27.8) | 15 (34.1) | 0.62 c |
| Bradycardia | 14 (77.8) | 31 (70.5) | 0.75 d |
| Cutaneous necrosis | 1 (5.6) | 0 | -- |
aValues are expressed as No. (%).
bP values less than 0.05 were considered statistically significant.
c The results of the chi-square test.
d The results of Fisher’s exact test.
The in-hospital mortality rate was 11.1% and 44% in the intervention and control groups, respectively (P = 0.02). Abnormal MRI results were reported in 50% (n = 8) of 16 patients in the intervention group with available brain MRI, as well as in 5 patients of the control group among 13 neonates in the control group with available brain MRI (P > 0.05).
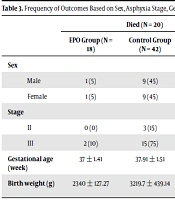
The frequency of the primary and secondary outcomes (mortality of the whole study population and MRI findings of the intervention group) categorized based on the study variables is shown in Table 3. As demonstrated, most of the deceased neonates had stage III HIE, and most of the neonates with stage II HIE recovered (P = 0.002). Also, deceased neonates had a lower GA (P = 0.008; Table 3). The MRI findings were not different according to the studied variables in the intervention group (P > 0.05; Table 4 and supplementary table).
| Died (N = 20) | Recovered (N = 42) | P Value b | |||||
|---|---|---|---|---|---|---|---|
| EPO Group (N = 18) | Control Group (N = 42) | Total | EPO Group (N = 18) | Control Group (N = 42) | Total | ||
| Sex | 0.37 c | ||||||
| Male | 1 (5) | 9 (45) | 10 (0.50) | 10 (23.8) | 16 (38.09) | 26 (61.9) | |
| Female | 1 (5) | 9 (45) | 10 (0.50) | 6 (14.28) | 10 (23.08) | 16 (38.09) | |
| Stage | 0.002 d | ||||||
| II | 0 (0) | 3 (15) | 3 (15) | 9 (21.42) | 15 (35.71) | 24 (57.14) | |
| III | 2 (10) | 15 (75) | 17 (85) | 7 (16.6) | 11 (26.19) | 18 (42.85) | |
| Gestational age (week) | 37 ± 1.41 | 37.91 ± 1.51 | 37.85 ± 1.5 | 38.62 ± 1.40 | 39.13 ± 1.51 | 38.94 ± 1.51 | 0.008 e |
| Birth weight (g) | 2340 ± 127.27 | 3219.7 ± 439.14 | 3131.7 ± 496.7 | 3108.8 ± 418.53 | 3065.6 ± 530.70 | 3082.02 ± 48 6.08 | 0.71 e |
aValues are expressed as No. (%) or mean ± SD.
bP values less than 0.05 were considered statistically significant.
c The results of the chi-square test.
d The results of Fisher’s exact test.
e The results of the student t-test.
aValues are expressed as No. (%) or mean ± SD.
b P values less than 0.05 were considered statistically significant.
c The results of the chi-square test.
d The results of the student t-test.
The a-EEG results showed moderate, severe, and severe abnormal changes with seizures in 11.1% (n = 2), 77.8% (n = 14), and 5.6% (n = 1) of the patients in the intervention group, respectively; 1 patient had unclear results (5.6%). In the intervention group, 2 patients with severe abnormal EEG died. The survived newborns had normal a-EEG results or moderate abnormal changes at discharge.
5. Discussion
In the present study, the comparison of 2 groups with similar demographics, including sex distribution, mean BW, GA, and Apgar score, showed that administering 6 doses of EPO to neonates during head cooling could significantly reduce the in-hospital mortality rate from 44% to 11%, although other variables, such as mean LOS, frequency of ventilator requirement, the incidence of thrombocytopenia, bradycardia, and seizures were similar between the study groups. These results showed a significant effect of EPO on HIE when used in addition to TTM. Although hypothermia is introduced as the standard treatment to control HIE, it is unfortunately not effective in some cases, resulting in remarkable mortality and morbidity in the affected neonates (21). The results of a meta-analysis of 3072 neonates with HIE estimated the mortality rate at 39% following TTM (22), which is close to the mortality rate of the control group in the present study. In a randomized controlled trial (RCT) by Zhou et al., a comparison of the outcomes between neonates undergoing selective head cooling and the control group showed a significantly lower mortality rate in the intervention group (20% vs 29%) (23), which confirms the efficacy of TTM. However, the mortality rate of this study was much lower than that of the control group in our study. In another RCT by Jacobs et al., a whole-body cooling (WBC) resulted in a mortality rate of 51.4% (9), which is higher than that of the control group in our study. This discrepancy among the results of the studies can be because of the effect of several factors that influence the efficacy of TTM, such as the temperature and duration of cooling (24).
Additionally, the present study showed that the addition of EPO to local hypothermia significantly reduced the short-term mortality rate of term neonates with HIE. The EPO dosage and administration method used in the present study were according to the results of a phase I trial, reporting that this dose was sufficient for the optimal plasma EPO levels for neuroprotection (25). The results of the present study are in line with the previous evidence on the efficacy of EPO in improving the outcomes of neonates with HIE; however, the treatment details and studied outcomes differed (21, 26). In the study by Zhu et al., 300 or 500 IU/kg administered every other day for 2 weeks to neonates with moderate to severe HIE resulted in 24.6% mortality, which was significantly lower than the mortality rate of neonates who received conventional treatment (43.8%) (27). These results are in line with that suggested by the present study in terms of the effect of EPO on reducing neonatal mortality. However, we used EPO as adjunctive therapy in addition to TTM in the intervention group, while they used it as a separate treatment. Moreover, the results of our study showed that the mean BW of deceased neonates who died was significantly lower, and most of the neonates with stage III HIE had death outcomes, indicating the role of other factors in the mortality of neonates with HIE. Badr-El Din et al. confirmed the short-term efficacy and safety of adjunctive EPO therapy (500 IU/kg every other day for 2 weeks) used along with WBC on newborns with HIE, which resulted in shorter LOS, decreased incidence of clinically detectable seizures, and improved radiological and short-term clinical outcomes (17). However, in our study, EPO had no effects on LOS and the incidence of seizures in neonates with HIE. This difference in the results can be attributed to the different dosages, and TTMs used. As the previous studies have used different doses and administration methods, more studies are required in this regard to determine the most appropriate dose with the highest safety and efficacy.
Rogers et al. used four different doses of EPO on 22 neonates, and the results showed that none died, and 36% had moderate to severe brain injury based on MRI results (18), which is similar to that in our study, reporting abnormal MRI results in 50% of patients in the intervention group. However, they reported no death, while we observed death in 11% of our patients. This difference could be attributed to the different doses of EPO used. Rogers et al. reported 1 case with moderate to severe developmental disorder (cerebral palsy in 4 limbs), associated with bilateral damage to the basal ganglia and thalamus (18), which was also observed in 1 patient in our study; the cerebral parenchyma echogenicity found in periventricular areas was also similar to that reported by Rogers et al. (18). Elmahdy et al. also showed no differences in brain MRI results, EEG results, and frequency of seizure between the EPO (2500 IU/kg subcutaneous EPO for 5 days) and non-EPO groups (19), which is consistent with the results of the present study. However, they did not use EPO as an adjunctive therapy like us. Similar to our study, Wu et al. suggested multiple high doses of IV EPO (1000 U/kg for 7 days) combined with TTM as a safe treatment with further neuroprotection, decreased severity of brain injury on MRI, and improved short-term motor outcomes in neonates with moderate/severe HIE during a 1-year follow-up (28), which is in line with the general results of the present study, although they did not report a death. Other studies have also confirmed the effect of TTM on reducing brain injury, detected in MRI results of the neonates, especially in the most commonly affected area, basal ganglia (29, 30), confirming the low rate of abnormal MRI results in the control group of our study, although some MRI results were inaccessible in our study. As the frequency of sentinel events, the severity of cerebral palsy and mortality rate differ based on the pattern of brain injury (31). More studies are required to determine the effect of the interventions on patients’ brain injuries.
The mechanisms of the neuroprotective effect of EPO are not fully understood, while the suggested mechanisms include reducing neuronal apoptosis (32), inflammation (33), oxidative injury (34), glutamate toxicity (35), and enhanced angiogenesis (36). Future studies are needed to address the heterogeneous timing and the neuroprotective mechanisms of EPO that contribute to neonatal brain injury in HIE. Moreover, because of the different findings on the rate of adverse outcomes of HIE in patients who underwent EPO therapy, additional studies are required to determine whether EPO can effectively improve each outcome or not.
One of the main strengths of this study was the comparison of short-term consequences between 2 matched groups. Among the limitations of the present study, one can name the small sample size, lack of simultaneous intervention, and non-randomized assignment of subjects to the groups. Furthermore, due to the short-term nature of the assessments, the results of the present study cannot be generalized to longer periods.
5.1. Conclusions
Administration of high-dose EPO along with head cooling reduced the short-term mortality rate in neonates with HIE compared with those treated with head cooling alone (without EPO). However, it did not influence the neonates’ LOS, frequency of seizure, mechanical ventilation, and side effects of treatments. More studies are required to investigate the most effective dose of EPO as adjunctive therapy for HIE in a large sample of neonates with long-term follow-up.