1. Background
Upper gastrointestinal bleeding (UGIB) is an uncommon but life-threatening condition in the pediatric population. The UGIB encompasses bleeding originating from the esophagus to the ligament of Treitz (1). The source of UGIB could be in the esophagus, stomach, or duodenum. Bleeding from other sources, such as the pancreas or biliary system, may occur but is not common (2). The mortality rate for pediatric UGIB has a range of 5% - 15% or more in developing countries based on various properties of populations that experience different conditions associated with UGIB, such as acute variceal hemorrhage (1, 3, 4).
Management of UGIB consists of fluid resuscitation, hemodynamic stabilization, blood transfusion for unstable patients, and pharmacotherapy. Proton pump inhibitors (PPIs), vasoconstrictive agents, and octreotide have been used for patients with UGIB (2). Octreotide, a somatostatin synthetic peptide analog, mimics the pharmacologic activity of somatostatin with advantages in pharmacokinetics parameters. It is a good alternative for somatostatin due to its potency and longer half-life (5). The octreotide mechanism of action and its pharmacologic activity is to decrease the production of gastrointestinal (GI) peptides, namely gastrin, cholecystokinin, and secretin via binding to the G protein-coupled receptors (6, 7). Octreotide has been widely administered in acute variceal bleeding treatment (8). However, it has been utilized less frequently to manage UGIB by other etiologies. Most clinical trials involving octreotide have been performed in adults (9). To our knowledge, no clinical trial has investigated octreotide efficacy in pediatric non-variceal UGIB. Few reports exist on octreotide efficacy in the pediatric population (10, 11).
2. Objectives
In this placebo-controlled randomized clinical trial, we aimed to evaluate octreotide efficacy and safety in treating non-variceal UGIB as an add-on therapy to PPIs in the pediatric population.
3. Methods
This prospective randomized, double-blind placebo-controlled clinical trial was performed on patients with acute non-variceal UGIB aged 1 - 15 years referred to Mofid Children's Hospital affiliated to Shahid Beheshti University of Medical Sciences, Tehran, Iran, a tertiary pediatric medical center, during February 2019 - December 2019. The study was conducted in accordance with the declaration of Helsinki and was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran, and was registered in the Iranian Registry of Clinical Trials (IRCT20120415009475N6). Written informed consents were obtained from the legal guardian of all the patients before enrollment.
The patients were randomly selected and divided into test and control groups. Permuted block randomization method was applied, and randomization sequence was generated using sealed envelope randomization service and online databases for clinical trials, London, United Kingdom. The study was designed as a double-blinded placebo-controlled clinical trial, and both the administrator and the analyzer were blinded. All octreotide and placebo vials were similar in shape and label, so they were not distinguishable from each other. The medication and placebo were provided in coded packages, and the codes were based on the permuted block randomization sequence provided to the researcher by a designated person.
Patients with a diagnosis of non-variceal UGIB, as well as with normal serum glutamic oxaloacetic transaminase, glutamic pyruvic transaminase, alkaline phosphatase, and bilirubin, normal coagulation profile [i.e., international normalization ratio (INR)], and with an age range of 1 - 15 years were enrolled in the study. The exclusion criteria were variceal UGIB, hepatic impairment defined as Child-Pugh category C (12), chronic liver disease, symptomatic hepatosplenomegaly, platelet count < 30000 cell/mL, and unstable hemodynamic despite maximum hydration and blood transfusion if indicated (13). The patients were first examined for hemodynamic status and initial resuscitation considering correct fluid and electrolyte. The included patients received pantoprazole at the dose of 1 mg/kg/day (max = 8 mg/kg/h) for gastric pH of 4 or more for at least 24 h and octreotide at the dose of 1 µg/kg/h titrated to response (max = 2 µg/kg/h) concomitantly.
All patients were investigated for the time of bleeding discontinuation as the primary outcome of the study. Secondary outcomes were the need for blood transfusion and hemoglobin value-related severe adverse drug reaction (ADR) considered as safety outcomes. The demographic, clinical, and preclinical data were collected under the supervision of a clinical pharmacist. The recorded demographic data included age, gender, weight, and height for all the patients. Moreover, clinical data about the chief complaints of patients, physician diagnosis of non-variceal UGIB, underlying disease and conditions, coagulation profile, GI problems, and endoscopic results were recorded. Laboratory data, including complete blood count, liver function, and enzymes tests, were also recorded. In addition, daily pantoprazole and octreotide doses were recorded with ongoing therapy.
3.1. Statistical Analysis
All statistical analyses were performed using the statistical package for the social sciences (SPSS) version 20.0 software. Mean and standard deviation were used to describe continuous variables and numbers, and the percentage was applied to express categorical variables. A paired t-test and a chi-square test were utilized to examine the quantitative and qualitative data, respectively. P-value < 0.05 was considered statistically significant.
4. Results
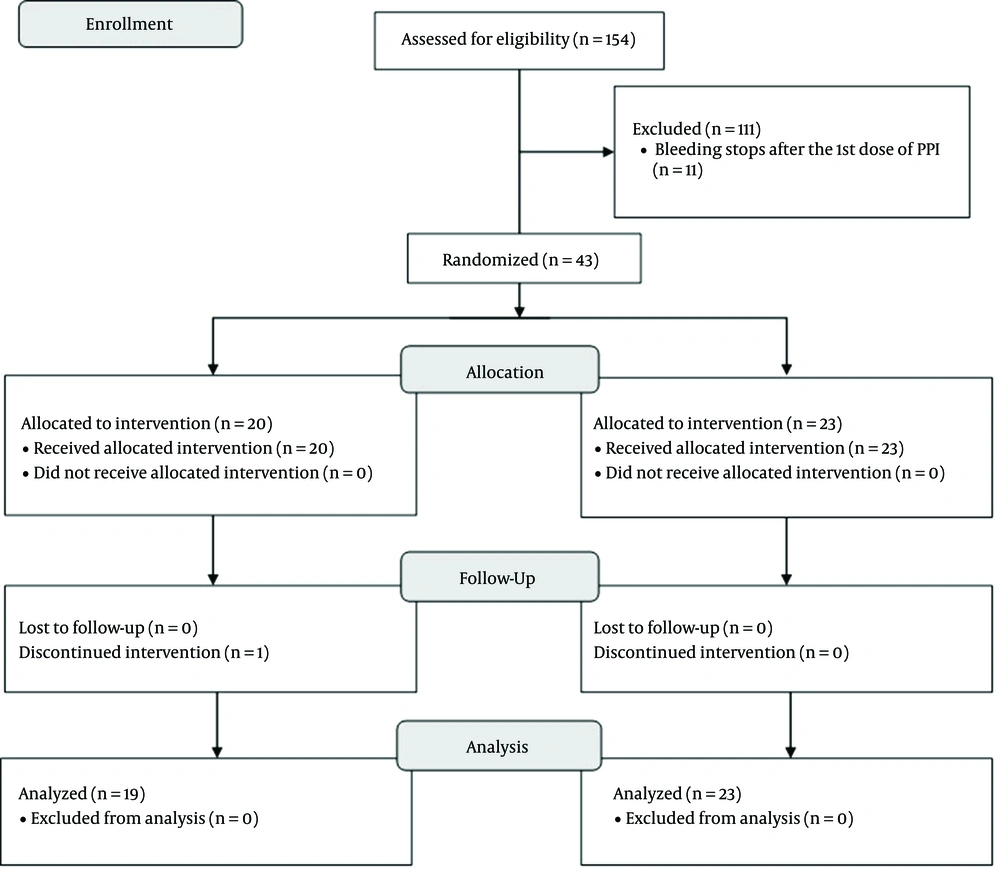
In this study, 26 male and 17 female participants were recruited. The median age of the patients was 4 (IQR = 5) years. The distribution of gender, age, and other demographic data were not significantly different between the two research groups. The study flow diagram is represented in Figure 1. The patients received treatment for a median duration of 7 days (maximum ten days). The demographic values of the recruited participants are presented in Table 1. Non-variceal acute GI bleeding (AGIB) was diagnosed for all the patients by a pediatric gastroenterologist. All the cases were hemodynamically stable during the investigation. Hematemesis, melena, and coffee ground vomiting were the most frequent chief complaints. There were no statistically significant differences between the intervention and control groups in terms of patients’ complaints at the first presentation.
| Value | Intervention Group (n = 20) | Control Group (n = 23) | P-Value |
|---|---|---|---|
| Gender, No. (%) | 0.5 | ||
| Male | 9 (45) | 8 (34.78) | |
| Female | 11 (55) | 15 (65.22) | |
| Age (y) | 4.25 (IQR = 4) | 4 (IQR = 4.7) | 0.386 |
| Weight (kg) | 21.23 ± 14.24 | 16.36 ± 8.91 | 0.215 |
| Height (cm) | 111.29 ± 50.17 | 114.18 ± 89.90 | 0.604 |
| Past medical history (3 most common) (%) | 0.053 | ||
| None | 4 (20) | 15 (65.22) | |
| Chemotherapy | 5 (25) | 0 (0) | |
| NSAID usage | 2 (10) | 3 (13.04) | |
| Prior GI bleeding | 1 (5) | 2 (8.7) | |
| Presenting chief complaint (3 most common) (%) | 0.416 | ||
| Hematemesis | 7 (35) | 11 (47.83) | |
| Melena | 4 (20) | 4 (17.39) | |
| Coffee ground emesis | 2 (10) | 2 (8.7) |
Abbreviations: NSAID, non-steroidal anti-inflammatory drugs; GI, gastrointestinal.
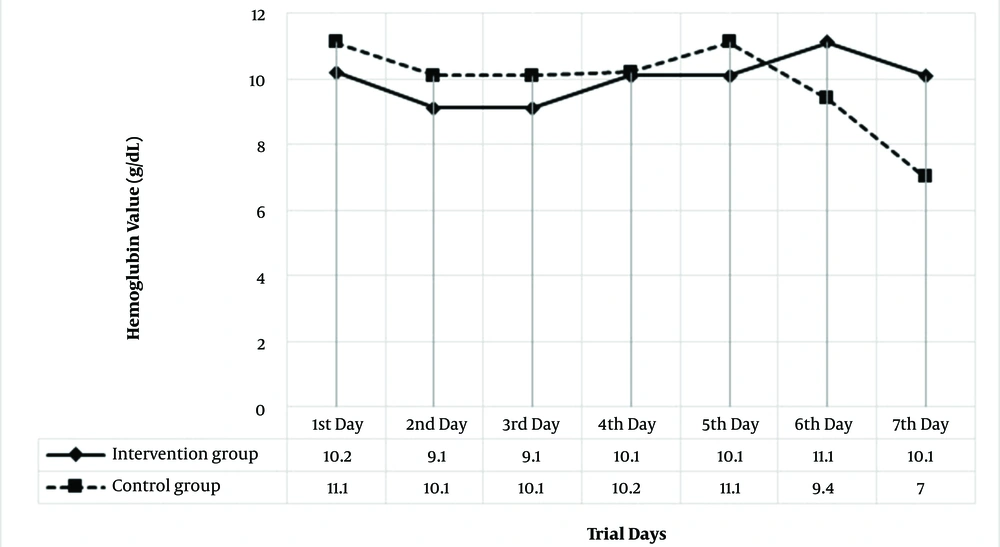
The GI bleeding mainly occurred in patients with no past medical history, followed by patients with a history of receiving chemotherapy or non-steroidal anti-inflammatory drugs and those diagnosed with AGIB. The two groups were not significantly different regarding past medical history distribution (P = 0.052). Twenty-seven of the subjects underwent the endoscopic procedure. Normal GI mucosa was observed in eight patients, and ten of the patients were diagnosed with gastritis. Moreover, nine patients were diagnosed with antral ulcers, gastric ulcers, and duodenitis. The endoscopic findings were not different between the two groups (P = 0.381). However, GI bleeding did not stop in one patient in the intervention group despite receiving treatment with pantoprazole and octreotide, and an invasive procedure was performed. Moreover, one patient in the test group who underwent chemotherapy for hematologic malignancy died because of severe infection. Baseline hemoglobin values were not different between the two groups (10.2 ± 0.03 vs. 11.1 ± 6.75, P = 0.08). However, hemoglobin drops were significantly more in the control group (P = 0.014). Figure 2 demonstrates hemoglobin values in the two groups. None of the patients in the two groups had coagulopathy.
The mean INR of the recruited patients was 1.08 ± 0.22. Participants in the intervention group received 15 (IQR = 26) mL/kg of packed red blood cells, and in the control group, they received 10 (IQR = 11.00) mL/kg to correct severe anemia in the bleeding condition. No significant difference was observed between the two groups regarding packed red blood cells (P = 0.056).
Table 2 demonstrates octreotide doses in the test group for a maximum of ten days of treatment. The time of GI bleeding resolving was not significantly different between the two groups (60.47 ± 00.74 vs. 59.87 ± 00.91, P = 0.99). Concerning ADRs, no patient receiving octreotide showed ADR (14) consistent with severe conditions, such as hypersensitivity reactions, cholelithiasis, pancreatitis, or hyperglycemia. Alteration in the heart rate and rhythm, such as QTc interval prolongation or any arrhythmias, did not occur in patients receiving octreotide. Hepatomegaly, splenomegaly, and rise in hepatic aminotransferases were not observed in any patients.
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | Day 10 |
|---|---|---|---|---|---|---|---|---|---|
| 10 (34) | 17 (20) | 17 (20) | 12.5 (16) | 10 (13.5) | 10 (28.5) | 10 (27) | 10 (35) | 10 (18) | 6 (20) |
a Median (interquartile range).
5. Discussion
In this clinical trial, octreotide add-on therapy to pantoprazole was not associated with a decrease in the duration of bleeding. Medication administration did not result in a reduced need for blood transfusion in this population. Octreotide as a peptide could alter various aspects of physiologic pathways in the GI tract and has a valuable therapeutic role as add-on therapy in the pharmacotherapy of a variety of GI disorders. In adults, approved octreotide indications include vasoactive intestinal peptide tumors, treatment for the management of esophageal variceal bleeding, secretory or chemotherapy-induced diarrhea, excessive ileostomy losses, gastroenteropancreatic neuroendocrine tumors, and pancreatitis (15, 16). In the pediatric population, octreotide is mainly used for secretory diarrhea or variceal GI bleeding (17). No clinical trial has investigated the safety and efficacy of octreotide in the pharmacotherapy of AGIB for the pediatric population without esophageal varices.
In an eighteen-month survey of the adult population, patients admitted to the hospital with the diagnosis of AGIB underwent the endoscopic procedure. Patients with variceal bleeding and those undergoing surgical procedures were excluded from the study. Next, the patients were randomly assigned to receive 50 mg ranitidine every 8 h alone or 100 mg octreotide every 8 h subcutaneously concomitant with ranitidine. Based on the pathologic data from endoscopic findings, no differences were observed between the two groups of patients in this study (18).
Similarly, blood transfusion and the length of hospital stay were not different between the two groups. A major part of the data in our study was extracted from adult studies as there is no data on the use of octreotide in the pediatric population. However, based on the pharmacokinetic data in children, it is known that octreotide clearance is more rapid than adults, and we should use it as intravenous infusion but not at bolus doses. In limited retrospective studies in children, it was shown that octreotide might be beneficial in controlling non-arterial and also variceal GI bleeding in children, but not in bleeding from mucosal ulcers (19).
In a prospective nonrandomized clinical study on the safety and efficacy of octreotide in controlling acute upper GI bleeding, all patients received octreotide for 5 days. Twenty-two patients had non-variceal bleeding confirmed by endoscopic evidence (20). In contrast to our study, GI bleeding did not stop in about one-third of patients in the latter investigation (20). Patient population, dosing, and the length of therapy were different between the two groups. In a survey of three children with chronic hepatic impairment, octreotide was efficacious in controlling bleeding from portal hypertension, unknown origin, or arteriovenous malformations. The three children received octreotide at the dose of 4 - 8 µg/kg/day, which led to bleeding cessation and hemoglobin rise in the first week (21). Our study did not observe hepatic impairment from octreotide administration or significant elevation of hepatic aminotransferases. The GI bleeding is more frequent in critically ill patients than in other cases (22). However, acute GI bleeding with clinical symptoms, including hemodynamic instability, decreased hemoglobin to 2 g/dL, and less frequent blood transfusion. In developing countries, GI bleeding mainly occurs due to GI varices. The PPIs are the mainstay of non-variceal GI bleeding treatment. Recently published studies on GI bleeding in children showed that octreotide administered concomitantly with a PPI would not affect bleeding, as in our study (2).
Considering no differences in baseline values in our research, patients receiving octreotide showed a significantly higher hemoglobin value than the controls. In a study conducted at Alberta Pediatric Hospital during January 1998 - December 2004, octreotide was used in children for different purposes (11). Among 21 patients receiving octreotide, eleven cases received octreotide for massive GI bleeding. The causes of GI bleeding in these patients included esophageal and gastric varices, portal hypertension, and gastropathy. Octreotide was administered in these patients at the dose of 2.2 ± 1 mg/kg/h, tapered to the half dose after 24 h, and discontinued after the bleeding stopped. In this retrospective study (11), cardiovascular adverse events and hyperglycemia were the most common ADRs. Moreover, in this prospective randomized clinical trial, we did not observe any ADR related to octreotide consumption in pediatric patients. However, we recorded hypertension in one patient admitted to the hospital due to hypertension under treatment by labetalol. Nevertheless, it was not distinguishable that this hypertension was octreotide ADR or resulted from uncontrolled hypertension. A critical issue about octreotide safety in the pediatric population is that most ADRs reported in studies are observed in patients receiving octreotide for longer than our study. For example, in the study in Alberta, patients received octreotide for 7 - 90 days which was longer than our study in which patients received octreotide for only 10 days. In investigating pediatric population, performing studies with large sample size and multicentric trials are difficult. The current study had some limitations, including recruiting patients in the Gastroenterology Ward, which affects the study population, difficulty in the diagnosis of non-variceal bleeding before diagnostic endoscopy and its influence on patient recruitment, limited sample size, single-centered study design, not monitoring ADR after discharging the patients, and not performing diagnostic endoscopy procedures for all the patients.
5.1. Conclusions
According to the findings of our study, octreotide did not reduce the bleeding duration and blood transfusion rate in patients with non-variceal AGIB. However, the hemoglobin value was significantly higher in patients receiving octreotide than others. No serious ADR leading to drug cessation was reported. Further investigations are required concerning the usage of octreotide due to the high cost and poor efficacy of this medication in pediatric patients. Moreover, it is recommended to perform multicentered, prospective, randomized trials with a larger sample size to evaluate the safety and efficacy of octreotide in the pediatric population.