1. Background
The removal of residual hydronephrosis after successful pyeloplasty is not common in long-term evaluations. Therefore, for the success of surgery after pyeloplasty in ureteropelvic junction obstruction (UPJO), it is always necessary to use nuclear scans for patients. Nuclear scans for annual follow-up are costly and have radiation for patients. The anteroposterior diameter (APD) of the pelvis on ultrasound and renogram scan is the most commonly used investigation for follow-up after pyeloplasty; however, APD has some limitations, such as operator mutability and very slow enhancement. Therefore, the present study aimed to use new ultrasound parameters for the follow-up of patients after pyeloplasty.
The sensitivity of ultrasound in the diagnosis of obstruction increases, compared to scintigraphy as the gold standard, when other criteria are used in follow-up, including the thickness of the renal cortex. Increased cortical thickness after pyeloplasty can be a sign of surgical success. Two new parameters have recently been identified. One is the pelvis-cortex (P/C) ratio (1), and the other is the percentage of pelvic improvement (PI) in APD (2).
2. Objectives
With the help of this study, it was intended to achieve a way for postsurgery monitoring of pyeloplasty patients with ultrasound alone.
3. Methods
In this study, the data of patients (age range: 0 - 14 years) who underwent pyeloplasty for UPJO in Mofid Children Hospital Pediatric surgery ward, Tehran, Iran, from 2017 to 2021 were analyzed, and those children were included whose preoperative and postoperative ultrasounds and renal scans were accessible.
The inclusion criteria were as follows:
(1) Unilateral cases
(2) The absence of renal abnormalities, such as high-grade urinary reflux (grades VI and V), obstructive megaureter, duplicated ureter, horseshoe kidney, and posterior urethral valve
(3) The contribution of the cases in follow-up after operation at least one year
(4) No history of kidney operations
(5) The presence of the diuretic renal scan before surgery and 6 months after surgery with standard conditions, including adequate hydration and transurethral catheter during the procedure
(6) A history of dismembered Anderson-Hynes pyeloplasty
(7) The insertion of a double J stent in the anastomosis place for one month
(8) Ultrasound performed in the third and sixth months after surgery and then annually
The following parameters were measured in ultrasound:
(1) Renal pelvic APD
(2) Length of kidney
(3) The P/C ratio
(4) The PI in APD
The exclusion criteria were as follows:
(1) No obviously obstructed renography scans (tracer clearance half-time < 20 minutes)
(2) Kidney crossed vessels
(3) Failed pyeloplasty that underwent redo procedure
(4) Bilateral renal disease
(5) The presence of other kidney-associated anomalies
(6) The absence of ultrasound findings
(7) Loss to follow-up (< 1 year)
By definition (2), APD (anteroposterior pelvis) incomes the transverse area districted among the anterior and posterior pelvis wall of the kidney that is enclosed by the boundaries of the renal parenchyma and separated from the extrarenal pelvis. This calculation helps avoid the shrinkage influence of the renal pelvis on the defined parameter. Romao et al. (2) defined the formula for the calculation of PI in APD as follows:
The variables used in this study were age at surgery, gender, presence of clinical signs and symptoms before surgery, above-mentioned ultrasound criteria (i.e., APD, P/C ratio, and PI in APD), improvement in hydronephrosis, renal function before and after surgery, and improvement in isotope clearance. The preoperative diuretic clearance half-time could not be measured quantitatively for technical reasons (most with diuretic scans where results over 40 minutes were not quantified). Categorical variables are expressed as frequencies. All data analysis was checked with SPPS software (version 17). Quantitative data are described by the mean and standard deviation (SD). Receiver operating characteristic (ROC) curve analysis was applied to recognize predictive accuracy.
4. Results
For 73 children with unilateral UPJO, pyeloplasty was performed in 4 years (2017 - 2021). For this study, 67 cases met all the inclusion criteria. The mean age at operation was 30 ± 37.44 months (range: From 2 months to 13 years; 55.2% of patients operated before 1 year of age). Moreover, 83.6% of the patients were male (male to female ratio: 5.1:1). Additionally, 65.7% of the subjects were diagnosed with prenatal ultrasound, and 15% of the cases had kidney-associated anomalies, the most common of which was mild vesicoureteral reflux in 4 patients (6%). Other associated anomalies included kidney stones, hemophilia, undescended testicles, and neuropathic bladder.
Symptomatic hydronephrosis was recognized in 27 cases (40.3%), such as cyclic pain (n = 17), acute abdominal pain (n = 4), urinary tract infection (UTI) (n = 2), abdominal mass (n = 1), hematuria (n = 1), and irritability (n = 1). All operations were performed with one surgeon. The type of UPJO was documented as intrinsic (adynamic) in 64 cases (95.5%), and the others were external pressure in 2 cases (3%) and intraluminal in 1 case (1.5%). Moreover, the affected side was reported as the left and right in 59.7% (n = 40) and 40.3% (n = 27) of the patients, respectively. Blood pressure before surgery based on age was normal in 65 (97%) cases, and only 2 patients (3%) had high pressure. Table 1 shows the mean values of pelvic APD, kidney cortex diameter, P/C ratio, and preoperative split renal function before surgery.
| Variables | Values |
|---|---|
| Mean age at operation (mon) | 30 ± 37.4 |
| Gender (male) | 56 (83.6) |
| Male to female ratio | 5.1:1 |
| Prenatal diagnosis | 65.7% |
| Associated anomaly | 15% |
| Symptomatic hydronephrosis | 27 (40.3) |
| Affected side (left) | 40 (59.7) |
| Mean anteroposterior diameter of the pelvis before surgery (mm) | 33.93 ± 11.47 |
| Mean kidney cortex diameter before surgery (mm) | 5.26 ± 2.07 |
| Mean pelvis-cortex ratio before surgery | 7.56 ± 4.38 |
| Mean preoperative split renal function | 42.23 ± 12.68 |
| Preoperative tracer clearance half-time (min) | 15.1 ± 37.54; (range: 0 - 228) |
a Values are expressed as mean ± standard deviation or No. (%).
Dimercaptosuccinic acid scan before surgery was decreased in 65 cases and was normal in 2 cases. The mean preoperative tracer clearance half-time in diuretic renography was 15.1 minutes (SD = 37.54; range; 0 - 228 minutes). Preoperative tracer clearance half-time in diuretic renography (T1/2 preoperative) in 75.8% 0f patients (n = 50) was 0 because the complete obstruction was between 21 - 228 minutes. A voiding cystourethrogram before surgery was performed in all cases, and only 4 cases (6/1%) had a low grade of reflux. Table 1 shows the characteristics of patients before surgery.
All patients underwent dismembered reduction pyeloplasty. In all cases, a double J stent was inserted during the operation. The mean follow-up time was 32 ± 10.56 months (range: 12 - 48 months). Table 2 shows the mean values of APD, kidney cortex diameter, P/C ratio, and PI in APD 3 and 6 months after surgery. The mean postoperative split renal function was documented at 41.84% (SD = 12.82%; range: 5 - 63%). The mean postoperative tracer clearance half-time in diuretic renography was 20.77 minutes (SD = 3.56; range: 12 - 40 minutes). The complications after surgery were documented in two patients (3%), one case with an anastomotic stricture that underwent redo surgery and another with UTI that underwent conservative management.
| Variables | Mean ± SD (Range) |
|---|---|
| Follow-up duration (mo) | 32 ± 10.56 (12 - 48) |
| Anteroposterior diameter 3 months after surgery (mm) | 18.1 ± 7.55 (3 - 46) |
| Kidney cortex diameter 3 months after surgery (mm) | 6.72 ± 2.03 (2.7 - 12) |
| Pelvis-cortex ratio 3 months after surgery (mm) | 3.09 ± 2.03 (3 - 9.1) |
| Pelvic improvement (percentage) in anteroposterior diameter 3 months after surgery, % | 43.29 ± 21.38 (0 - 90) |
| Anteroposterior diameter 6 months after surgery | 15.43 ± 6.01 (2 - 32.5) |
| Kidney cortex diameter 6 months after surgery (mm) | 7.24 ± 2.21 (2.7 - 12) |
| Pelvis-cortex ratio 6 months after surgery | 2.8 ± 3.93 (2 - 32.5) |
| Pelvic improvement (percentage) in anteroposterior diameter 6 months after surgery, % | 50.83 ± 20.22 (0 - 96) |
| Postoperative split renal function, % | 41.84 ± 12.82 (5 - 63) |
| Postoperative tracer clearance half-time (min) | 20.77 ± 3.56 (12 - 40) |
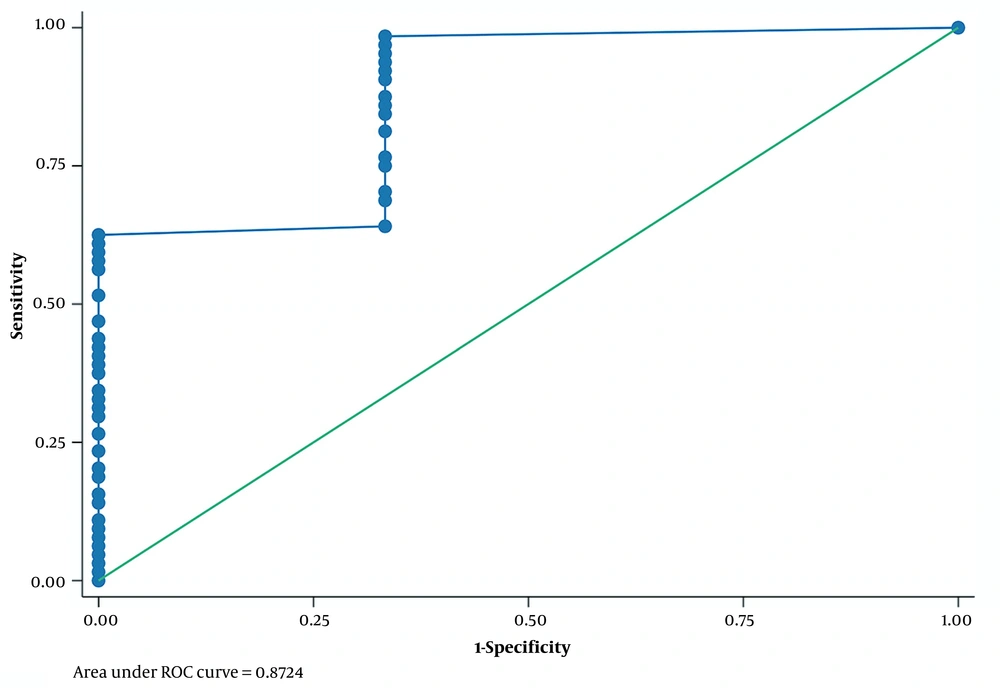
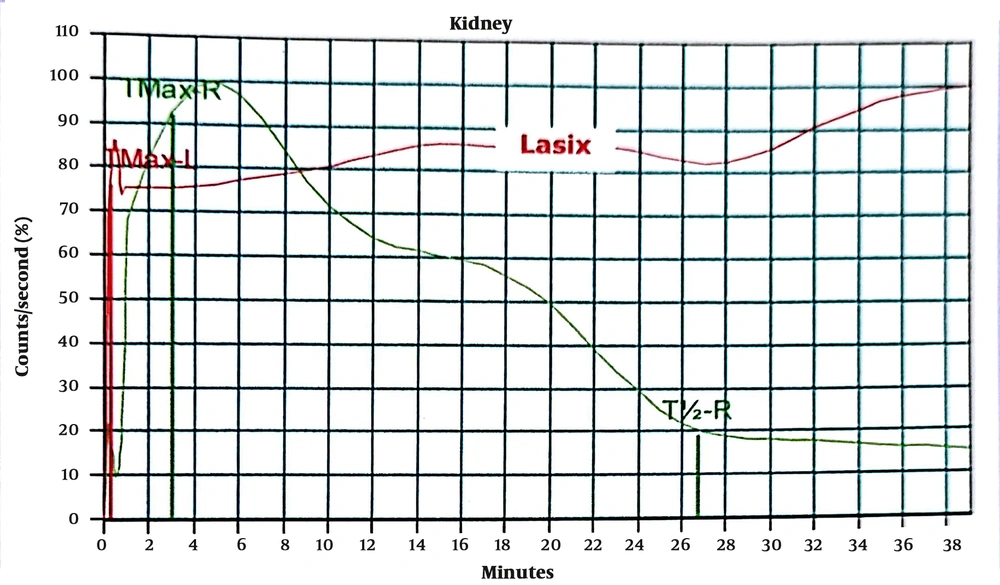
The ROC curve analysis was performed for the diagnostic utility of the studied parameters for successful pyeloplasty. In ROC curve analysis, it was observed that PI in APD > 12% 3 months after surgery versus diethylene triamine pentaacetic acid (DTPA) 6 months after surgery could predict successful pyeloplasty with sensitivity, specificity, and area under the curve (AUC) equal to 98.44%, 66.67%, and 0.87, respectively (Table 3; Figure 1).
| Cutpoint | Sensitivity, % | Specificity, % | LR+ | LR- |
|---|---|---|---|---|
| (≥ 0) | 100.00 | 0.00 | 1 | |
| (≥ 12) | 98.44 | 66.67 | 2.9531 | 0.0234 |
| (≥ 13) | 96.88 | 66.67 | 2.9062 | 0.0469 |
| (≥ 16) | 95.31 | 66.67 | 2.8594 | 0.0703 |
| (≥ 17) | 93.75 | 66.67 | 2.8125 | 0.0938 |
| (≥ 18) | 92.19 | 66.67 | 2.7656 | 0.1172 |
| (≥ 20) | 90.63 | 66.67 | 2.7187 | 0.1406 |
| (≥ 21) | 87.50 | 66.67 | 2.625 | 0.1875 |
| (≥ 25) | 85.94 | 66.67 | 2.5781 | 0.2109 |
| (≥ 26) | 84.38 | 66.67 | 2.5312 | 0.2344 |
| (≥ 28) | 81.25 | 66.67 | 2.4375 | 0.2813 |
| (≥ 29) | 76.56 | 66.67 | 2.2969 | 0.3516 |
| (≥ 30) | 75.00 | 66.67 | 2.25 | 0.375 |
| (≥ 32) | 70.31 | 66.67 | 2.1094 | 0.4453 |
| (≥ 33) | 68.75 | 66.67 | 2.0625 | 0.4688 |
| (≥ 34) | 64.06 | 66.67 | 1.9219 | 0.5391 |
| (≥ 35) | 62.50 | 100.00 | 0.375 | |
| (≥ 36) | 60.94 | 100.00 | 0.3906 | |
| (≥ 37) | 59.38 | 100.00 | 0.4063 | |
| (≥ 39) | 57.81 | 100.00 | 0.4219 | |
| (≥ 40) | 56.25 | 100.00 | 0.4375 | |
| (≥ 43) | 51.56 | 100.00 | 0.4844 | |
| (≥ 46) | 46.88 | 100.00 | 0.5313 | |
| (≥ 48) | 43.75 | 100.00 | 0.5625 | |
| (≥ 50) | 42.19 | 100.00 | 0.5781 | |
| (≥ 51) | 40.63 | 100.00 | 0.5938 | |
| (≥ 52) | 39.06 | 100.00 | 0.6094 | |
| (≥ 53) | 37.50 | 100.00 | 0.625 | |
| (≥ 54) | 34.38 | 100.00 | 0.6563 | |
| (≥ 55) | 32.81 | 100.00 | 0.6719 | |
| (≥ 57) | 31.25 | 100.00 | 0.6875 | |
| (≥ 58) | 29.69 | 100.00 | 0.7031 | |
| (≥ 60) | 26.56 | 100.00 | 0.7344 | |
| (≥ 62) | 23.44 | 100.00 | 0.7656 | |
| (≥ 63) | 20.31 | 100.00 | 0.7969 | |
| (≥ 66) | 18.75 | 100.00 | 0.8125 | |
| (≥ 67) | 15.63 | 100.00 | 0.8438 | |
| (≥ 70) | 14.06 | 100.00 | 0.8594 | |
| (≥ 71) | 10.94 | 100.00 | 0.8906 | |
| (≥ 74) | 9.38 | 100.00 | 0.9063 | |
| (≥ 76) | 7.81 | 100.00 | 0.9219 | |
| (≥ 82) | 6.25 | 100.00 | 0.9375 | |
| (≥ 84) | 4.69 | 100.00 | 0.9531 | |
| (≥ 85) | 3.13 | 100.00 | 0.9688 | |
| (≥ 90) | 1.56 | 100.00 | 0.9844 | |
| (> 90) | 0.00 | 100.00 | 1 |
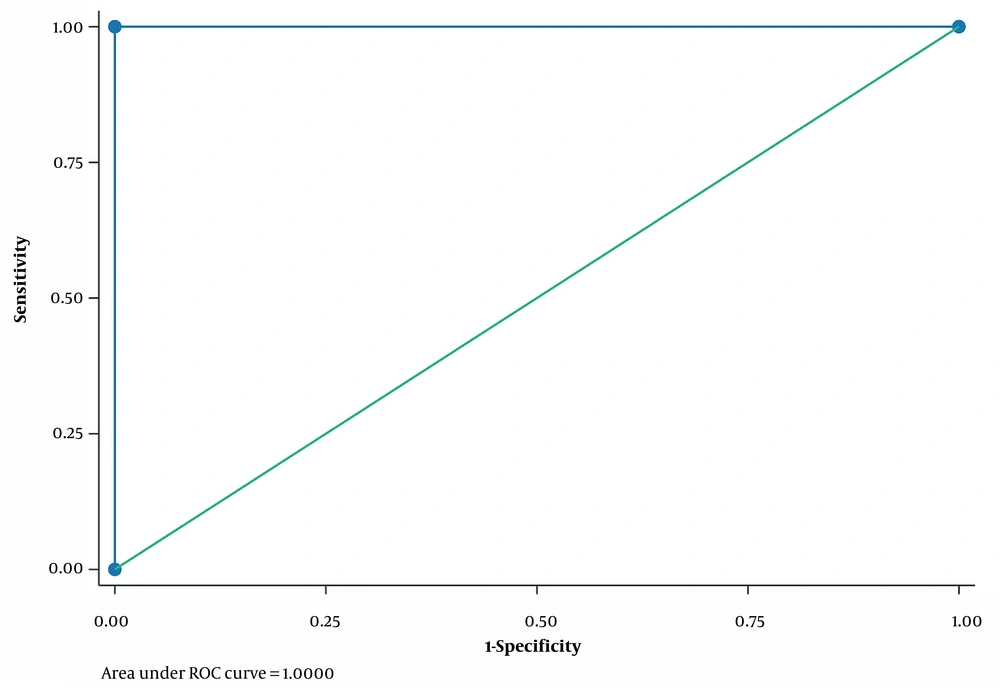
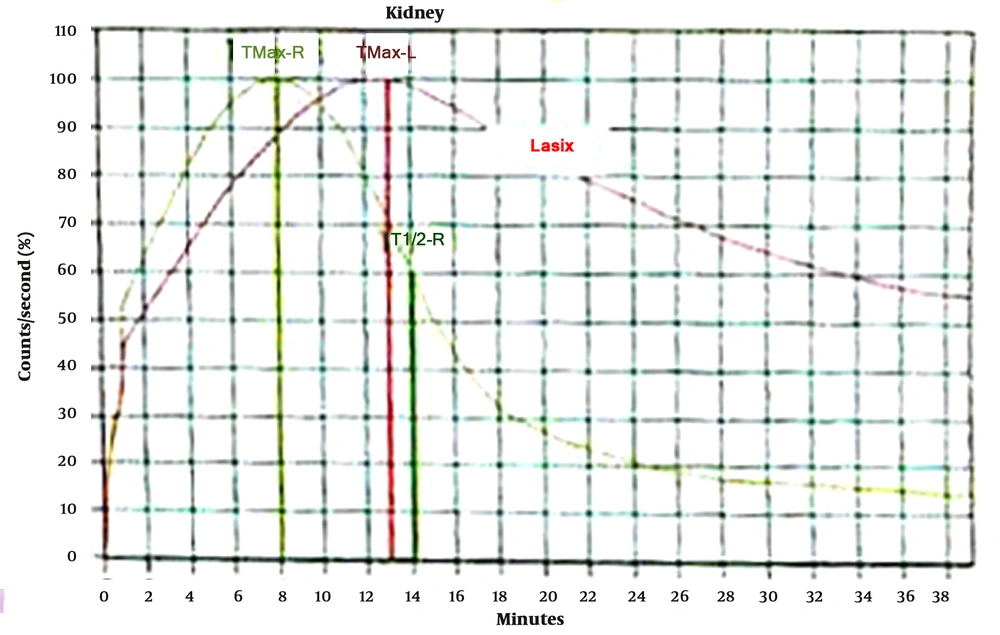
In ROC curve analysis, it was observed that PI in APD > 26% 6 months after surgery versus DTPA 6 months after surgery could strongly predict successful pyeloplasty with sensitivity and specificity of 100% and AUC of 1 (Table 4; Figure 2).
| Cutpoint | Sensitivity, % | Specificity, % | LR+ | LR- |
|---|---|---|---|---|
| (≥ 0) | 100.00 | 0.00 | 1 | |
| (≥ 26) | 100.00 | 100.00 | 0 | |
| (≥ 28) | 96.88 | 100.00 | 0.0313 | |
| (≥ 29) | 95.31 | 100.00 | 0.0469 | |
| (≥ 30) | 89.06 | 100.00 | 0.1094 | |
| (≥ 32) | 85.94 | 100.00 | 0.1406 | |
| (≥ 34) | 81.25 | 100.00 | 0.1875 | |
| (≥ 35) | 79.69 | 100.00 | 0.2031 | |
| (≥ 36) | 78.13 | 100.00 | 0.2188 | |
| (≥ 39) | 75.00 | 100.00 | 0.25 | |
| (≥ 40) | 71.88 | 100.00 | 0.2813 | |
| (≥ 41) | 70.31 | 100.00 | 0.2969 | |
| (≥ 42) | 67.19 | 100.00 | 0.3281 | |
| (≥ 43) | 65.63 | 100.00 | 0.3438 | |
| (≥ 44) | 59.38 | 100.00 | 0.4063 | |
| (≥ 45) | 54.69 | 100.00 | 0.4531 | |
| (≥ 46) | 53.13 | 100.00 | 0.4688 | |
| (≥ 47) | 50.00 | 100.00 | 0.5 | |
| (≥ 48) | 48.44 | 100.00 | 0.5156 | |
| (≥ 50) | 43.75 | 100.00 | 0.5625 | |
| (≥ 52) | 42.19 | 100.00 | 0.5781 | |
| (≥ 53) | 40.63 | 100.00 | 0.5938 | |
| (≥ 54) | 37.50 | 100.00 | 0.625 | |
| (≥ 55) | 35.94 | 100.00 | 0.6406 | |
| (≥ 56) | 26.56 | 100.00 | 0.7344 | |
| (≥ 60) | 23.44 | 100.00 | 0.7656 | |
| (≥ 61) | 20.31 | 100.00 | 0.7969 | |
| (≥ 62) | 18.75 | 100.00 | 0.8125 | |
| (≥ 63) | 15.63 | 100.00 | 0.8438 | |
| (≥ 65) | 14.06 | 100.00 | 0.8594 | |
| (≥ 66) | 10.94 | 100.00 | 0.8906 | |
| (≥ 70.7) | 9.38 | 100.00 | 0.9063 | |
| (≥ 71) | 7.81 | 100.00 | 0.9219 | |
| (≥ 74) | 6.25 | 100.00 | 0.9375 | |
| (≥ 75) | 4.69 | 100.00 | 0.9531 | |
| (≥ 76) | 1.56 | 100.00 | 0.9844 | |
| (≥ 77) | 0.00 | 100.00 | 1 | |
| (≥ 80) | 100.00 | 0.00 | 1 |
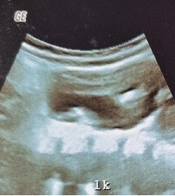
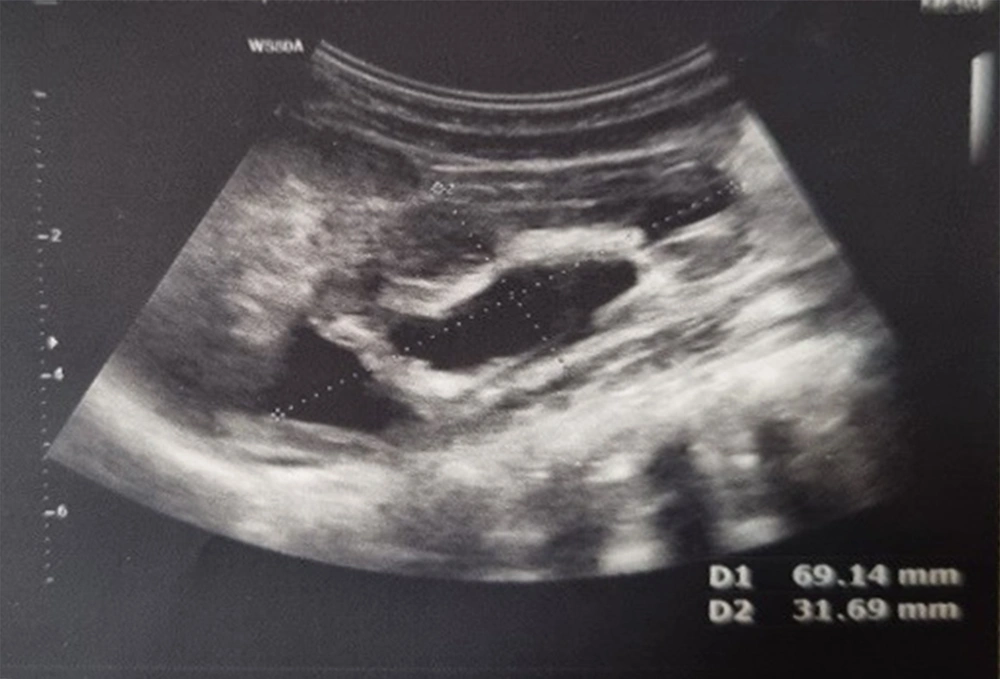
The above-mentioned consequences showed that the two PI in APD 3 and 6 months after the operation could provide valuable results. Therefore, renal scans can alert if there is noticeable PI in APD on follow-up. The percentage of PI in APD in 3 and 6 months after surgery was the most reliable variable to predict successful pyeloplasty (AUC = 0.87 and AUC = 1); nevertheless, postoperative APD 6 months after surgery alone was not a good predictor (AUC = 0.06). The PI in APD of 26% or higher 6 months after surgery was associated with success with sensitivity and specificity of 100% (AUC = 1). Cortex depth 6 months after surgery versus DTPA 6 months after surgery were also reliable indices (AUC = 0.78). In this study, the P/C ratio 3 and 6 months after surgery versus DTPA 6 months after surgery was not suitable for the evaluation of successful pyeloplasty (AUC = 0.26 and AUC = 0.12). Figures 3 to 6 illustrate ultrasound and DTPA in patients before surgery and 6 months after surgery.
5. Discussion
Pelvic APD diameter and renal scintigraphy play a major role in the diagnosis and postoperative follow-up following UPJO. However, the APD is not a dependable item owing to several factors, such as the hydration status of the patient, compliance of renal pelvis, and type of pyeloplasty. With the help of this study, it was intended to achieve a way for postsurgery monitoring of pyeloplasty patients with ultrasound alone. The present study showed that PI in APD of 26% or higher 6 months after surgery was associated with success with sensitivity and specificity of 100%. Cortex depth 6 months after surgery versus DTPA 6 months after surgery were also reliable indices.
The items that are of particular importance after surgery include changes in the degree of hydronephrosis and fluctuations in parameters, such as APD, and changes in renal function (3, 4). These parameters can be used to track the outcome of surgery. Diuretic renography is usually performed after pyeloplasty about 3 to 6 months after surgery (5, 6). In various studies in different references, there is disagreement on the timing of ultrasound after surgery. Numerous authors recommend stopping follow-up after 2 years of surgery (7).
Almodhen et al. (8) used postoperative diuretic scan results with postoperative ultrasound in 101 pyeloplasty patients. None of the patients who had hydronephrosis after surgery needed re-pyeloplasty. this study showed that ultrasound is highly sensitive in showing postoperative obstruction. However, as it is known, nuclear scans for annual follow-up are costly and have radiation for patients. Similarly, Cost et al. (9) described ultrasound as a valuable and reliable monitoring method in patient follow-up. Only 28% of patients with recurrent hydronephrosis required re-pyeloplasty in the third month after surgery. In both reports, only the Society of Fetal Urology grade of hydronephrosis was used. However, in recent studies, the parameters mentioned in ultrasound will be used in the study. The sensitivity of ultrasound in the diagnosis of obstruction increases, compared to scintigraphy as the gold standard, when other criteria are used in follow-up, including the thickness of the renal cortex. Increased cortical thickness after pyeloplasty can be a sign of surgical success. Two new parameters have recently been identified. One is the P/C ratio (1), and the other is the percentage of PI in APD (2). In this study, the aforementioned parameters were used to provide suitable guidance for follow-up.
A series of studies by Chipde et al. (10) and Longpre et al. (11) showed that a high percentage of patients after pyeloplasty avoided diuretic isotope renography if the ultrasound parameters did not get worse. This issue can save time and money and avoid infections caused by catheterization for scanning and radiation from the follow-up scan.
In a study by Fernandez-Ibieta et al. (12), it was shown that all children who had obstruction after reoperation had a PI < 15%, which could indicate the presence of obstruction with 100% sensitivity at 3 and 6 months. Therefore, if the disease reaches a PI above 15% after surgery, there will be no need for nuclear scan studies. The aforementioned study demonstrated that by the end of the first year after surgery, there were ultrasound changes, and the ultrasound changes remained unchanged in the second year after surgery. In the current study, the PI in APD of 26% or higher 6 months after surgery was associated with success with sensitivity and specificity of 100% (AUC = 1). Cortex depth 6 months after surgery versus DTPA 6 months after surgery were also reliable indices (AUC = 0.78).
Gharpure et al. (13) reported calyx to parenchymal ratio (CPR) as a good predictor for follow-up after pyeloplasty. This ratio is measured by ultrasound in the coronal view. The aforementioned study evaluated the utility of CPR in the follow-up of pyeloplasty with the APD of the pelvis and renal scintigraphy. The aforementioned study showed that CPR was an important prognosticator of surgical consequence with an accuracy of about 95.1%, and alteration in CPR was reported as an improved success factor after pyeloplasty as related to change in APD with an accuracy of about 85.2% (P = 0.01). Nevertheless, in the present study, the P/C ratio 3 and 6 months after surgery versus DTPA 6 months after surgery was not also suitable for the evaluation of successful pyeloplasty (AUC = 0.26 and AUC = 0.12).
Kljucevsek and Kljucevsek (14) used contrast‐enhanced percutaneous nephrosonography (cePNS) as an ultrasound contrast mediator ordered through the catheter in the kidney for the evaluation of the urinary tract patency in pediatric patients. They used nine cePNS in seven patients to assess the urinary tract patency before additional management. The technical achievement rate and accuracy of cePNS inspections were 100%. The aforementioned study reported that CePNS is a radiation‐free technique and can be applied as a continuance of ultrasound guides. However, these models of study are not usual and need more reports.
Rickard et al. (15) evaluated the renal parenchyma-to-hydronephrosis area ratio to discover the upgrade or deterioration of hydronephrosis after surgical intervention. The aforementioned study’s data proposed that this item can provide an objective valuation for enhancement after the operation, compared to other usual ultrasounds. Nonetheless, the current study showed that cortex depth 6 months after surgery versus DTPA 6 months after surgery was also not a reliable index.
In Tc-99m-mercaptoacetyltriglycine (MAG3) renography slow drainage does not unavoidably describe obstruction. Obstruction means fighting urinary flow and urinary stasis at the ureteropelvic junction, thereby injuring the affected kidney (16). Kiblawi et al. assessed the efficiency of ultrasound for the evaluation of decompensated urinary drainage during the early follow-up of patients After pyeloplasty. The aforementioned study reported that postoperative reduction in renal pelvis diameter is enough to rule out the reappearance of obstruction. A renal scan appears to be indicated only in patients with a postoperative rise in the APD of the renal pelvis on ultrasound (17).
Burgu et al. (18), in a randomized study, compared the findings of ultrasound and nuclear renography in children with a history of pyeloplasty without considering pelvic reduction. In the aforementioned study, 42 cases with prenatally unilateral hydronephrosis were encompassed. Moreover, 20 children randomly underwent pyeloplasty with pelvic reduction, and 22 cases underwent pelvis sparing pyeloplasty. The children were assessed with ultrasound scans on the first, third, and sixth months after surgery and MAG3 scans 6 months after surgery. The mean follow-up duration was 37 ± 5.6 weeks.
The anteroposterior length of the pelvis diminished significantly in the group with pelvic reduction contrast compared to the pelvis-sparing group, in the first-month ultrasounds (i.e., first and third months after surgery). However, the alteration was not important in the sixth month. Renal washout time (T½) in MAG3 renography was significantly decreased in the pelvic reduction group. Differential renal function was not affected after pelvic reduction.
The perfect period of follow-up of pediatric patients after pyeloplasty has mainly remained unknown. Unfortunately, no study has shown standard guidelines to assess a suitable duration and kind of follow-up after pyeloplasty. Numerous centers reported that the mainstream of unsuccessful pyeloplasty cases is identified within 3 years after repair. Younger children with severe hydronephrosis and those with a history of open technique are inclined to be followed up for an extended time. Most cases with recurrent obstruction were shown with severe hydronephrosis before surgery (19, 20).
Rickard et al. (21) reported single-center pyeloplasty information in 151 cases. Only children with a complete database of APD dimensions were encompassed (n = 138). The subjects were divided into three PI-APD groups, namely < 20%, 20 - 39%, and > 40%. Of 138 cases, 6 patients (4%) underwent redo surgery for UPJO. The aforementioned study concluded that PI in APD greater than 40% at the first visit (3 months) after surgery powerfully expects pyeloplasty success, and up to 82% of the subjects presented resolved hydronephrosis. The data of the current study suggests that up to 85% of renography cases in groups with < 20% PI-APD led to redo surgery. Rickard M. recommended PI-APD as a hopeful strategy to decrease radiation contact of children after pyeloplasty.
In ROC curve analysis, it was observed that the percentage of PI in APD > 26% 6 months after surgery versus DTPA 6 months after surgery could strongly predict successful pyeloplasty with sensitivity and specificity of 100% and AUC of 1. Without standard guidelines for children after pyeloplasty, the length of follow-up and perfect imaging after repair are amendable and vary according to the individual’s desire. Therefore, a general standard guideline is needed, using randomized controlled multicenter trials, to resolve these serious requests. Several modalities are utilized for UPJO postoperative follow-up. In addition, the misuse and or overuse of these modalities not only increases the treatment cost but also is unethical. The best way to prevent these issues is the adoption of guidelines. The guidelines can help with surgeons’ views to properly apply these modalities, as shown in the precise antibiotic utilization and prescription (22, 23).
5.1. Conclusions
The comparison of the reliability of kidney ultrasound findings after pyeloplasty versus kidney scans for success in patients with UPJO showed that the percentage of PI in postoperative APD can offer that children require more monitoring with other investigations throughout follow-up as guidance. The present study identified that the percentage of PI in APD > 26% 6 months after surgery can strongly predict successful pyeloplasty and is a strong predictor of surgical outcome. Unnecessary repeated nuclear scans 6 months after surgery can be avoided using the aforementioned parameter. The present study recommended the use of PI in APD in the routine preoperative practice and postoperative follow-up of children that have undergone pyeloplasty.