1. Background
Inappropriate drug administration in pediatric patients has become a global issue in public health. Irrational prescription and medication errors put pediatric patients at a higher risk of unwanted side effects than adults (1). In pediatrics, medication errors have a high potential for harm and are life treating in some patients (2). Then, preventing medication errors becomes a big deal worldwide in the clinical workflow.
Despite advances in the diagnosis and treatment of diseases, pediatric mortality rates are still high. Pediatric patients differ from adults in many aspects of medication therapy, such as tolerance and taste priority. They also vary from adults in pharmacokinetic and pharmacodynamic features, including drug metabolism and renal clearance (3, 4). To ensure the appropriate treatment in pediatric patients, there must be a need for different prescription methods, doses, or instructions. Despite numerous available pharmaceutical products, the Food and Drug Administration (FDA) has only approved a quarter of them for use in children. However, due to a lack of pediatric medicine needs, licensed drugs for adults are being used off-label (5-8). Drug management in pediatric patients is complex and insecure because limited information is available to validate stability, bioavailability, pharmacokinetics, pharmacodynamics, dosage precision, tolerance, and rebuilding ability (5, 6).
Studies show that medication misuse in children can lead to severe consequences (9-11). However, a primary source of information on the safety of drug prescription in children was prepared and published in 2014 by a group of French researchers entitled Pediatrics: Omission of Prescriptions and Inappropriate Prescriptions (POPI) (12). The POPI is the first screening tool to detect inappropriate prescriptions (IPs) (79 propositions) and omissions (25 propositions) in pediatrics based on essential criteria.
The POPI standard has been developed based on the available evidence of pediatric health problems and published articles (13). Because pediatric disorders differ from those afflicting the elderly, the classification of propositions under these criteria is based on the situations of children (14). The compilers of this list used a screening tool of older persons’ prescriptions (STOPP)/screening tool to alert to right treatment (START) proposition, which was provided with the same format for adults as their work pattern. The only other tool for rational prescription in pediatrics is the modified POPI (the United Kingdom) tool which provided a list of potentially IPs and omissions for children in the UK (15, 16) for use in all pediatric practice settings. Another indicator for potential IPs to children was developed entirely in primary care settings (17, 18).
It would be reasonable to modify POPI to be (10) applicable in each country due to particular variations in the incidence of disease, the supply of various formularies, and, therefore, the diversity in pediatric practice. Consequently, the present study pursued modifying the POPI tool for use in Iran’s pediatric practice in inpatient and outpatient settings by adjusting it according to Iranian clinical guidelines.
2. Objectives
The current study was designed to assess the applicability of the POPI tool to practice outside France by matching the propositions to the Iran medicine list and clinical guidelines. The current study also aimed to modify the tool required for application in Iran’s pediatric practice, thereby facilitating the supplementary assessment of the tool using Iranian prescribing data.
3. Methods
3.1. Cross-cultural Adaptation and Validation Process
The POPI indicators were translated into Farsi according to the recommended method by the World Health Organization. For this purpose, forward- and backward-translation procedures were used. Two forward translators created the target language version, and one backward translator recreated the source version. A healthcare professional linguistic expert on the target language helped achieve a qualified cross-cultural adaptation process. Then, the checklists were matched to common medications in Iran and the INF.
Propositions on the IP list were submitted for validation to experts during the agreement survey. The experts in this study comprised pediatricians, pediatric pulmonologists, neonatologists, clinical pharmacists, pediatric nephrologists, pediatric cardiologists, pediatric endocrinologists, pediatric gastroenterologists, and pediatric hematologists/oncologists.
A two-round Delphi survey was conducted in Mashhad and Tabriz, Iran, to determine the consensus on developing a new POPI. A Delphi questionnaire was prepared. A three-point Likert scale was used, and the experts were asked to explain why they agreed or disagreed with their chosen statement in the POPI proposition. This study retained the proposition with the agreement of > 70% of experts who had given a non-zero rating. A draft of a modified POPI for pediatric patients was developed following the preliminary conceptual checklist. This checklist is then turned from the questionnaire form to the instruction form.
3.2. Applicable Potential Modified POPI
The modified POPI was then piloted in a cross-sectional study. The population of the study was children under 12 years. With the help of the Social Security Organization and the Social Security Organization and Registration Office,
We randomly selected children (400 individuals in Mashhad and 1207 individuals in Tabriz) and their related prescriptions (2034 prescriptions in Mashhad and 7050 prescriptions in Tabriz) during the study period. They were evaluated in turn. In total, 9084 prescriptions were recruited as separate files. Then, for ease of data analysis, all the files were imported into an excel file one by one.
The used, modified POPI tool contained 98 propositions (Table 1). Some of these propositions, which were not applicable to this population (e.g., omission of prescriptions) or for which information was unavailable (e.g., body mass index, indication, organ function, and comorbidities) of the prescriptions, were not analyzed as part of the study. The total number of propositions analyzed for this study was 43 of the 98 propositions stated on the modified POPI tool (i.e., the Persian version).
| Symptoms | Original POPI Propositions | Agree Without Change | Agree with the Specified Change | Against | Modified POPI Proposition | Retained |
|---|---|---|---|---|---|---|
| Fever and pain | ||||||
| 1 | Prescription of two alternating antipyretics as a first-line treatment | 13/16 (81.25) | 3/16 (18.75) | 0/16 (0) | Retained | |
| 2 | Prescription of a medication other than paracetamol as a first-line treatment (for pain) (except in the case of migraine) (pain and fever) | 10/16 (62.5) | 4/16 (25) | 2/16 (12.5) | Retained | |
| 3 | Rectal administration of paracetamol as a first-line treatment | 3/16 (18.75) | 10/16 (62.5) | 3/16 (18.75) | In cases of intolerance to the oral type and the need to accelerate the response to the drug, it can be prescribed; however, generally, the oral type is preferred. | |
| 4 | Combined use of two NSAIDs | 10/16 (62.5) | 4/16 (25) | 1/16 (6.25) | Retained | |
| 5 | Oral solutions of ibuprofen administered in more than three doses per day using a graduated pipette of 10 mg/kg (other than Advil) (pain and fever) | 3/16 (18.25) | 8/16 (50) | 5/16 (31.25) | Misused more than four times | |
| 6 | Opiates to treat migraine attacks | 11/16 (68.75) | 4/16 (25) | 1/16 (6.25) | Retained | |
| 7 | Failure to give sugar solution to newborn babies and infants under 4 months 2 minutes prior to venipuncture | 8/16 (50) | 4/16 (25) | 4/16 (25) | Retained | |
| 8 | Failure to give an osmotic laxative to patients treated with morphine for more than 48 hours | 9/16 (56.25) | 4/16 (25) | 2/16 (12.5) | Retained | |
| Urinary infections | ||||||
| 9 | Nitrofurantoin as a prophylactic | 0/16 (0) | 1/16 (6.25) | 10/16 (62.5) | It is used as night prophylaxis, and the drug is discontinued if it has gastrointestinal side effects. | Removed |
| 10 | Nitrofurantoin as a curative agent in children under 6 years of age or any other antibiotic if avoidable | 10/16 (62.5) | 2/16 (12.5) | 4/16 (25) | Retained | |
| 11 | Antibiotic prophylaxis following an initial infection without complications (except in the case of uropathy) | 8/16 (50) | 4/16 (25) | 3/16 (18.75) | Retained | |
| 12 | Antibiotic prophylaxis in the case of asymptomatic bacterial infection (except in the case of uropathy) | 9/16 (56.25) | 5/16 (31.25) | 1/16 (6.25) | Retained | |
| Vitamin supplements and antibiotics prophylaxis | ||||||
| 13 | Fluoride supplements prior to 6 months of age | 11/16 (68.75) | 0/16 (0) | 2/16 (12.5) | Retained | |
| 14 | Insufficient intake of vitamin D; Minimum vitamin D intake: Breastfed baby: 1000 to 1 200 IU/day; Infant: 18 months of age (milk enriched in vitamin D): 600 to 800 IU/day; Children aged within 18 months to 5 years and adolescents aged within 10-18 years: Two quarterly; Loading doses of 80,000 to 100,000 IU/day in winter (adolescents can take this dose in one go) | 0/16 (0) | 14/16 (87.5) | 0/16 (0) | Inadequate intake of vitamin D3 (below the recommended dietary allowance); According to national guidelines: 5-day infant to 2-year children: 1 mL of A+D drops daily (400 IU/day); Children within 2-12 years: one pearl of 50,000 IU every 2 months; Adolescents 12-70 years: 50,000 IU every month; Over 70 years: 50,000 IU every 2 weeks | |
| 15 | Antibiotic prophylaxis with phenoxymethylpenicillin starting from 2 months of age and lasting until 5 years of age for children with sickle-cell anemia: 100,000 IU/kg/day (in two doses) for children weighing 10 kg or less and 50,000 IU/kg/day for children weighing over 10 kg (also in two doses) (vitamin supplements and antibiotic prophylaxis) | 7/16 (43.75) | 5/16 (31.25) | 0/16 (0) | This does not mean specific treatment with phenoxymethylpenicillin. Other well-known antibiotics might also be used. | |
| Mosquitos | ||||||
| 16 | Use of skin repellents in infants under 6 months and picaridin in children under 24 months | 12/16 (75) | 1/16 (6.25) | 3/16 (18.75) | Retained | |
| 17 | Anti-insect bracelets to protect against mosquitos and ticks | 11/16 (68.75) | 1/16 (6.25) | 4/16 (25) | Retained | |
| 18 | Ultrasonic pest control devices, vitamin B1, homeopathy, electric bug zappers, and sticky tapes without insecticide | 11/16 (68.75) | 2/16 (12.5) | 2/16 (12.5) | Retained | |
| 19 | DEET: 30% (max) before 12 years; 50% (max) after 12 years | 7/16 (43.75) | 4/16 (25) | 2/16 (12.5) | Removed | |
| 20 | IR3535: 20% (max) before 24 months; 35% (max) after 24 months | 6/16 (37.5) | 3/16 (18.75) | 2/16 (12.5) | Removed | |
| 21 | Mosquito nets and clothes treated with pyrethroids | 5/16 (31.25) | 0/16 (0) | 2/16 (12.5) | Removed | |
| Digestive problems (nausea, vomiting, diarrhea, and gastroesophageal reflux) | ||||||
| 22 | Metoclopramide | 4/16 (25) | 6/16 (37.5) | 1/16 (6.25) | Retained | |
| 23 | Domperidone | 3/16 (18.75) | 8/16 (50) | 5/16 (31.25) | Retained | |
| 24 | Oral administration of an intravenous proton pump inhibitor (notably by nasogastric tube) | 2/16 (12.5) | 9/16 (56.25) | 5/16 (31.25) | Retained | |
| 25 | Gastric antisecretory drugs to treat gastroesophageal reflux, dyspepsia, the crying of newborns (in the absence of any other signs or symptoms), and faintness in infants (nausea, vomiting, or gastroesophageal reflux) | 13/16 (81.25) | 1/16 (6.25) | 2/16 (12.5) | Retained | |
| 26 | Combined use of proton pump inhibitors and NSAIDs, for a short time, in patients without risk factors | 8/16 (50) | 5/16 (31.25) | 3/16 (18.75) | Retained | |
| 27 | Use of type H2 antihistamines for long periods of treatment (nausea, vomiting, or gastroesophageal reflux) | 14/16 (87.5) | 0/16 (0) | 2/16 (12.5) | Retained | |
| 28 | Erythromycin as a prokinetic agent (nausea, vomiting, or gastroesophageal reflux) | 5/16 (31.25) | 7/16 (43.75) | 4/16 (25) | Retained | |
| 29 | Use of setrons (5-HT3 antagonists) for chemotherapy-associated nausea and vomiting | 6/16 (37.5) | 7/16 (43.75) | 3/16 (18.75) | Retained | |
| 30 | Oral rehydration solution | 5/16 (31.25) | 4/16 (25) | 7/16 (43.75) | Retained | |
| 31 | Loperamide before 3 years of age (diarrhea) | 10/16 (62.5) | 4/16 (25) | 1/16 (6.25) | Retained | |
| Digestive problems (diarrhea) | ||||||
| 32 | Loperamide in the case of invasive diarrhea | 11/16 (68.75) | 1/16 (6.25) | 4/16 (25) | Retained | |
| 33 | Use of diosmectite (SMecta) in combination with another medication | 15/16 (93.75) | 0/16 (0) | 1/16 (6.25) | Retained | |
| 34 | Use of Saccharomyces boulardii (Ultra-levure) in powder form or in a capsule that has to be opened prior to ingestion to treat patients with a central venous catheter or an immunodeficiency | 6/16 (37.5) | 0/16 (0) | 0/16 (0) | Retained | |
| 35 | Intestinal antiseptics | 9/16 (56.25) | 3/16 (18.75) | 1/16 (6.25) | Retained | |
| 36 | Oral rehydration solution | 13/16 (81.25) | 3/16 (18.75) | 0/16 (0) | ||
| (ENT) Pulmonary problems (cough) | ||||||
| 37 | Pholcodine | 15/16 (93.75) | 1/16 (6.25) | 0/16 (0) | Retained | |
| 38 | Mucolytic drugs, mucokinetic drugs, or helicidine before 2 years of age | 15/16 (93.75) | 1/16 (6.25) | 0/16 (0) | Retained | |
| 39 | Alimemazine (theralene), oxomemazine (toplexil), and promethazine (e.g., phenergan and other types) | 11/16 (68.75) | 4/16 (25) | 1/16 (6.25) | Retained | |
| 40 | Terpene-based suppositories | 15/16 (93.75) | 1/16 (6.25) | 0/16 (0) | Retained | |
| 41 | Failure to propose a whooping cough booster vaccine for adults who are likely to become parents in the coming months or years (only applicable if the previous vaccination was more than 10 years ago) This booster vaccination should also be proposed to the family and the entourage of expectant parents (e.g., parents, grandparents, and nannies/childminders) | 10/16 (62.5) | 0/16 (0) | 0/16 (0) | Retained | |
| 42 | This booster vaccination should also be proposed to the family and the entourage of expectant parents (e.g., parents, grandparents, and nannies/childminders) (cough) | 12/16 (75) | 1/16 (6.25) | 0/16 (0) | Retained | |
| (ENT) Pulmonary problems (bronchitis in children) | ||||||
| 43 | Beta-2 agonists and corticosteroids to treat an infant’s first case of bronchiolitis | 5/16 (31.25) | 9/16 (56.25) | 2/16 (12.5) | Retained | |
| 44 | H1 antagonists, cough suppressants, mucolytic drugs, or ribavirin to treat bronchiolitis | 7/16 (43.75) | 6/16 (37.5) | 3/16 (18.75) | Retained | |
| 45 | Antibiotics in the absence of signs indicating a bacterial infection (e.g., acute otitis media or fever) | 9/16 (56.25) | 6/16 (37.5) | 1/16 (6.25) | Retained | |
| 46 | 0.9% NaCl to relieve nasal congestion (not applicable if nasal congestion is already treated with 3% NaCl delivered by a nebulizer) | 15/16 (93.75) | 0/16 (0) | 1/16 (6.25) | Retained | |
| 47 | Palivizumab in the following cases: 1, Neonates born both at under 35 weeks of gestation and under 6 months prior to the onset of a seasonal respiratory syncytial virus epidemic; 2, Children under 2 years who have received treatment for bronchopulmonary dysplasia in the past 6 months; 3, Children under 2 years suffering from congenital heart disease with hemodynamic abnormalities | 14/16 (87.5) | 1/16 (6.25) | 1/16 (6.25) | Retained | |
| (ENT) Pulmonary problems (ear, nose, and throat infections) | ||||||
| 48 | An antibiotic other than amoxicillin as a first-line treatment for acute otitis media, strep throat, or sinusitis (provided that the patient is not allergic to amoxicillin); an effective dose of amoxicillin for a pneumococcal infection is 80 - 90 mg/kg/day, and an effective dose for a streptococcal infection is 50 mg/kg/day | 10/16 (62.5) | 4/16 (25) | 2/16 (12.5) | Retained | |
| 49 | Antibiotic treatment for a sore throat, without a positive rapid diagnostic test result, in children under 3 years | 4/16 (25) | 1/16 (6.25) | 10/16 (62.5) | Retained | |
| 50 | Antibiotics for nasopharyngitis, congestive otitis, and sore throat before 3 years of age or laryngitis; antibiotics as a first-line treatment for acute otitis media showing few symptoms before 2 years of age | 13/16 (81.25) | 1/16 (6.25) | 2/16 (12.5) | Retained | |
| 51 | Antibiotics to treat OME, except in the case of hearing loss or if OME lasts for more than 3 months | 1/16 (6.25) | 9/16 (56.25) | 6/16 (37.5) | Retained | |
| 52 | Corticosteroids to treat acute suppurative otitis media, nasopharyngitis, or strep throat | 2/16 (12.5) | 6/16 (37.5) | 8/16 (50) | Retained | |
| 53 | Nasal or oral decongestants (e.g., oxymetazoline (aturgyl), pseudoephedrine (sudafed), naphazoline (derinox), ephedrine (rhinamide), tuaminoheptane (rhinofluimucil), and phenylephrine (humoxal)) | 13/16 (81.25) | 2/16 (12.5) | 1/16 (6.25) | Retained | |
| 54 | H1 antagonists with sedative or atropine-like effects (e.g., pheniramine and chlorpheniramine) or camphor; inhalers, nasal sprays, or suppositories containing menthol (or any terpene derivatives) before 30 months of age | 3/16 (18.75) | 8/16 (50) | 5/16 (31.25) | Retained | |
| 55 | Ethanolamine ténoate (rhinotrophyl) and other nasal antiseptics | 13/16 (81.25) | 3/16 (18.75) | Retained | ||
| 56 | Ear drops in the case of acute otitis media | 13/16 (81.25) | 1/16 (6.25) | Retained | ||
| 57 | Doses in mg for drinkable (solutions of) amoxicillin or josamycin | 11/16 (68.75) | 2/16 (12.5) | 2/16 (12.5) | Retained | |
| 58 | Paracetamol combined with antibiotic treatment for ear infections to relieve pain | 6/16 (37.5) | 4/16 (25) | 3/16 (18.75) | Retained | |
| (ENT) Pulmonary problems (asthma) | ||||||
| 59 | Ketotifen and other H1 antagonists and sodium cromoglycate | 11/16 (68.75) | 1/16 (6.25) | 3/16 (18.75) | Retained | |
| 60 | Cough suppressants | 2/16 (12.5) | 7/16 (43.75) | 7/16 (43.75) | Removed | |
| 61 | Asthma inhaler appropriate for the child’s age | 6/16 (37.5) | 8/16 (50) | 2/16 (12.5) | Retained | |
| 62 | Preventative treatment (i.e., inhaled corticosteroids) in the case of persistent asthma | 11/16 (68.75) | 3/16 (18.75) | 2/16 (12.5) | Retained | |
| Dermatological problems (acne vulgaris) | ||||||
| 63 | Minocycline | 9/16 (56.25) | 4/16 (25) | 3/16 (18.75) | Retained | |
| 64 | Isotretinoin, in combination with a member of the tetracycline family of antibiotics | 6/16 (37.5) | 3/16 (18.75) | 4/16 (25) | Retained | |
| 65 | Combined use of an oral and a local antibiotic | 7/16 (43.75) | 6/16 (37.5) | 0/16 (0) | Retained | |
| 66 | Cyproterone + ethinylestradiol (Diane-35) as a contraceptive to allow isotretinoin per os | 2/16 (12.5) | 8/16 (50) | 4/16 (25) | Removed | |
| 67 | Androgenic progestins (e.g., levonorgestrel, norgestrel, norethisterone, lynestrenol, dienogest, contraceptive implants, or vaginal rings) | 2/16 (12.5) | 8/16 (50) | 3/16 (18.75) | Retained | |
| 68 | Contraception (provided with a logbook/diary) for menstruating girls taking isotretinoin | 12/16 (75) | 0/16 (0) | 1/16 (6.25) | Retained | |
| 69 | Topical treatment (i.e., benzoyl peroxide, retinoids, or both) in combination with antibiotic therapy | 12/16 (75) | 0/16 (0) | 2/16 (12.5) | Retained | |
| Dermatological problems (scabies) | ||||||
| 70 | Application of benzyl benzoate (ascabiol) for periods longer than 8 hours for infants and 12 hours for children or for pregnant girls | 7/16 (43.75) | 5/16 (31.25) | 2/16 (12.5) | Retained | |
| 71 | A second dose of ivermectin 2 weeks after the first | 9/16 (56.25) | 2/16 (12.5) | 4/16 (25) | Retained | |
| 72 | Decontamination of household linen and clothes and treatment for other family members | 9/16 (56.25) | 2/16 (12.5) | 4/16 (25) | Retained | |
| Dermatological problems (lice) | ||||||
| 73 | Use of aerosols for infants, children with asthma, or children showing asthma-like symptoms, such as dyspnea | 12/16 (75) | 0/16 (0) | 3/16 (18.75) | Retained | |
| Dermatological problems (ringworm) | ||||||
| 74 | Treatment other than griseofulvin for Microsporum | 12/16 (75) | 0/16 (0) | 3/16 (18.75) | Retained | |
| 75 | Topical treatment combined with an orally-administered treatment | 5/16 (31.25) | 3/16 (18.75) | 5/16 (31.25) | Retained | |
| 76 | Griseofulvin during a meal containing a moderate amount of fat | 8/16 (50) | 3/16 (18.75) | 2/16 (12.5) | Retained | |
| Dermatological problems (impetigo) | ||||||
| 77 | Combination of locally applied and orally administered antibiotic | 13/16 (81.25) | 1/16 (6.25) | 2/16 (12.5) | Retained | |
| 78 | Fewer than twice per day for topical antibiotics | 6/16 (37.5) | 3/16 (18.75) | 6/16 (37.5) | Retained | |
| 79 | Any antibiotic other than mupirocin as a first-line treatment (except in cases of hypersensitivity to mupirocin) | 10/16 (62.5) | 3/16 (18.75) | 3/16 (18.75) | Retained | |
| Dermatological problems (herpes simplex virus) | ||||||
| 80 | Topical agents containing corticosteroids | 10/16 (62.5) | 4/16 (25) | 2/16 (12.5) | Retained | |
| 81 | Topical agents containing acyclovir before 6 years of age | 13/16 (81.25) | 3/16 (18.75) | 1/16 (6.25) | Retained | |
| 82 | Paracetamol during an outbreak of herpes | 6/16 (37.5) | 5/16 (31.25) | 5/16 (31.25) | Retained | |
| 83 | Orally administered acyclovir to treat primary herpetic gingivostomatitis | 9/16 (56.25) | 4/16 (25) | 2/16 (12.5) | Retained | |
| Dermatological problems (atopic eczema) | ||||||
| 84 | A strong dermocorticoid (clobetasol propionate, with 0.05% dermoval, and betamethasone dipropionate, with diprosone) applied to the face, armpits or groin, and the backside of babies or young children | 12/16 (75) | 3/16 (18.75) | 1/16 (6.25) | Retained | |
| 85 | More than once per day of a dermocorticoid, except in cases of severe lichenification | 14/16 (87.5) | 0/16 (0) | 1/16 (6.25) | Retained | |
| 86 | Local or systemic antihistamine during the treatment of outbreaks (atopic eczema) | 11/16 (68.75) | 3/16 (18.75) | 2/16 (12.5) | Retained | |
| 87 | Topically applied 0.03% tacrolimus before 2 years of age | 7/16 (43.75) | 6/16 (37.5) | 2/16 (12.5) | Retained | |
| 88 | Topically applied 0.1% tacrolimus before 16 years of age | 9/16 (56.25) | 3/16 (18.75) | 1/16 (6.25) | Retained | |
| 89 | Oral corticosteroids to treat outbreaks | 6/16 (37.5) | 4/16 (25) | 3/16 (18.75) | Retained | |
| Neuropsychiatric disorders (epilepsy) | ||||||
| 90 | Carbamazepine, gabapentin, oxcarbazepine, phenytoin, pregabalin, tiagabine, or vigabatrin in the case of myoclonic epilepsy | 7/16 (43.75) | 4/16 (25) | 4/16 (25) | Retained | |
| 91 | Carbamazepine, gabapentin, oxcarbazepine, phenytoin, pregabalin, tiagabine, or vigabatrin in the case of epilepsy with absence seizures (especially for childhood absence epilepsy or juvenile absence epilepsy) | 7/16 (43.75) | 5/16 (31.25) | 3/16 (18.75) | Retained | |
| 92 | Levetiracetam and oxcarbazepine in mL or in mg without systematically writing XX mg per Y mL | 10/16 (62.5) | 2/16 (12.5) | 1/16 (6.25) | Retained | |
| Neuropsychiatric disorders (depression) | ||||||
| 93 | A selective serotonin reuptake inhibitor antidepressant other than fluoxetine as a first-line treatment (in the case of pharmacotherapy) | 12/16 (75) | 0/16 (0) | 2/16 (12.5) | Retained | |
| 94 | Tricyclic antidepressants to treat depression | 8/16 (50) | 2/16 (12.5) | 5/16 (31.25) | Retained | |
| Neuropsychiatric disorders (nocturnal enuresis) | ||||||
| 95 | Desmopressin administered by a nasal spray | 4/16 (25) | 6/16 (37.5) | 5/16 (31.25) | Retained | |
| 96 | Desmopressin in the case of daytime symptoms | 8/16 (50) | 3/16 (18.75) | 4/16 (25) | Retained | |
| 97 | An anticholinergic agent used as a monotherapy in the absence of daytime symptoms | 11/16 (68.75) | 5/16 (31.25) | 0/16 (0) | Retained | |
| 98 | Tricyclic agents in combination with anticholinergic agents | 12/16 (75) | 3/16 (18.75) | 1/16 (6.25) | Retained | |
| 99 | Tricyclic agents as a first-line treatment | 10/16 (62.5) | 2/16 (12.5) | 3/16 (18.75) | Retained | |
| Neuropsychiatric disorders (anorexia) | ||||||
| 100 | Cyproheptadine (periactin) and clonidine | 6/16 (37.5) | 8/16 (50) | 1/16 (6.25) | Retained | |
| Attention deficit hyperactivity disorder with or without hyperactivity) | ||||||
| 101 | Pharmacological treatment before the age of 6 (before school), except in severe cases | 11/16 (68.75) | 4/16 (25) | 1/16 (6.25) | Retained | |
| 102 | Antipsychotic drugs to treat attention deficit disorder without hyperactivity | 10/16 (62.5) | 4/16 (25) | 2/16 (12.5) | Retained | |
| 103 | Slow-release methylphenidate in two doses per day rather than only one dose | 10/16 (62.5) | 2/16 (12.5) | 4/16 (25) | Retained | |
| 104 | Recording a growth chart (height and weight) if the patient is taking methylphenidate | 13/16 (81.25) | 1/16 (6.25) | 1/16 (6.25) | Retained |
Abbreviations: POPI, Pediatrics: Omission of Prescriptions and Inappropriate Prescriptions; NSAIDs, non-steroidal anti-inflammatory drugs; OME, otitis media with effusion.
a Values are expressed as No./total (%).
3.3. Statistical Methods and Sample Size
Data analysis was carried out through a Delphi study using qualitative methods and content analysis. Additionally, descriptive statistics were used to analyze the information related to IPs. As a sample, 9084 prescriptions were reviewed.
4. Results
Six propositions were omitted due to no existence in the INF or less popularity in Iran, lack of relevant national guidelines, or directly contradictory guidelines. At the end of the first round, 94% (98 of 104) of the submitted IP types were anticipated for scoring. All anticipated propositions obtained an agreement level of > 70% of experts. These propositions were retained, and five of them were reworded after the panel’s suggestions (i.e., propositions were modified to reflect national clinical guidance) (Table 1).
The experts were not solely satisfied with the existing statements in the POPI propositions. They provided a supplemental table of 12 potentially inappropriate drugs that should be used with caution due to their potential toxicity to children (Appendix 1). In the current analysis, only 10 of these propositions were used (prochlorperazine and quinupristin were not checked).
4.1. Assessment of Prescriptions Using the Modified POPI Tool
In Mashhad, 2034 prescriptions were registered during the year for 400 surveyed children, 203 (50.75%) and 197 (49.25%) of which were female and male patients, respectively. Their mean age was 6.25 years. After removing duplicate national numbers, out of 1560 children, 1207 remained in Tabriz. There were 530 females (43.91%) and 677 males (56.08%), with a mean age of 3.45 years. The age range studied in Tabriz differed from Mashhad in that the first group was under 1 year, the second group was within 1 - 4 years, and the third group was within 4 - 11 years.
The total number of medicines in Tabriz was 82537 over a year. Therefore, the average number of medicines per child is 18 drugs covered by insurance (and 3 drugs not covered by insurance). In Mashhad, 6003 medicines were registered for children over a year; therefore, each person receives an average of 15 drugs covered by insurance per year. At least, the rate of one inappropriate prescribed medication was 69% in Mashhad, almost twice that of Tabriz (35%).
Tables 2 and 3 summarize prescribing errors observed in pediatric prescriptions in Mashhad and Tabriz. The items marked as “not checked” were not reviewed. As shown in Table 2, in Mashhad, fluoroquinolones had the highest rate of IPs, followed by topical anesthetics, and tetracycline came in third place. In Tabriz, salbutamol had the highest rate of IPs, followed by selective serotonin reuptake inhibitors (SSRIs) and antihistamines.
| No. | Symptoms | Occurrence of Prescriptions in Mashhad, Iran | Occurrence of Prescriptions in Tabriz, Iran |
|---|---|---|---|
| 1 | Topical anesthetics (benzocaine, a mixture of lidocaine and prilocaine) | 12 (0.59) | Not checked |
| 2 | Ceftriaxone | 0 (0) | Not checked |
| 3 | Codeine | 0 (0) | Not checked |
| 4 | Diphenoxylate | 0 (0) | 0 (0) |
| 5 | Fluoroquinolones | 34 (1.67) | 0 (0) |
| 6 | Lindane | 2 (0.098) | 0 (0) |
| 7 | Selective serotonin reuptake inhibitors | 5 (0.25) | 12 (0.17) |
| 8 | Antihistamines | 11 (0.54) | 11 (0.16) |
| 9 | Salbutamol | Not checked | 299 (3.2) |
| 10 | Tetracycline | 14 (0.69) | 92 (1.3) |
a Values are expressed as No. (%).
| Inappropriate Prescriptions | Occurrence | |
|---|---|---|
| Mashhad | Tabriz | |
| 1. Prescription of two alternating antipyretics as a first-line treatment | 72 (3.54) | 358 (5.5) |
| 2. Prescription of a medication other than paracetamol as a first-line treatment (for pain) (except in the case of migraine) | 172 (8.46) | 22 (0.3) |
| 3. Rectal administration of paracetamol as a first-line treatment | 22 (1.08) | 174 (2.5) |
| 4. Combined use of two NSAIDs | 6 (0.30) | 1 (0.014) |
| 5. Metoclopramide | 81 (3.98) | 132 (1.9) |
| 6. Domperidone | 0 (0) | 0 (0) |
| 7. Oral administration of an intravenous proton pump inhibitor (notably by nasogastric tube) | 0 (0) | 0 (0) |
| 8. Gastric antisecretory drugs to treat gastroesophageal reflux, dyspepsia, crying of newborns (in the absence of any other signs or symptoms), and faintness in infants (nausea, vomiting, or gastroesophageal reflux) | 0 (0) | 4 (0.057) |
| 9. Combined use of proton pump inhibitors and NSAIDs, for a short period of time, in patients without risk factors | 0 (0) | 0 (0) |
| 10. Loperamide before 3 years of age | 0 (0) | 0 (0) |
| 11. Use of diosmectite in combination with another medication | 0 (0) | 0 (0) |
| 12. Opioid antitussive (codeine) | 19 (0.93) | 0 (0) |
| 13. Mucolytic drugs, mucokinetic drugs, or helicidine before 2 years of age | 87 (4.28) | 518 (7.3) |
| 14. Alimemazine, oxomemazine, and promethazine (and other types) | 15 (0.74) | 30 (0.43) |
| 15. Terpene-based suppositories | 0 (0) | 0 (0) |
| 16. Beta-2 agonists and corticosteroids to treat an infant’s first case of bronchiolitis | 0 (0) | 42 (0.6) |
| 17. H1 antagonists, cough suppressants, mucolytic drugs, or ribavirin to treat bronchiolitis | 0 (0) | 181(2.6) |
| 18. Antibiotics in the absence of signs indicating a bacterial infection (e.g., acute otitis media and fever) | 0 (0) | 842 (12) |
| 19. Antibiotics for nasopharyngitis, congestive otitis, sore throat before 3 years of age, or laryngitis; antibiotics as a first-line treatment for acute otitis media showing few symptoms before 2 years of age | 0 (0) | 0 (0) |
| 20. Corticosteroids to treat acute suppurative otitis media, nasopharyngitis, or strep throat | 241 (11) | 273 (3.9) |
| 21. Nasal or oral decongestants (i.e., oxymetazoline, pseudoephedrine, naphazoline, ephedrine, tuaminoheptane, and phenylephrine) | 109 (5.36) | 167 (2.4) |
| 22. H1 antagonists with sedative or atropine-like effects (i.e., pheniramine and chlorpheniramine) or camphor; inhalers, nasal sprays, or suppositories containing menthol (or any terpene derivatives) before 30 months of age | 0 (0) | 274 (3.9) |
| 23. Ethanolamine ténoate (rhinotrophyl) and other nasal antiseptics | 0 (0) | 0 (0) |
| 24. Ear drops in the case of acute otitis media | 0 (0) | 0 (0) |
| 25. Ketotifen and other H1 antagonists and sodium cromoglycate | 0 (0) | 89 (1.3) |
| 26. Cough suppressants | 0 (0) | 6 (0.085) |
| 27. Minocycline | 0 (0) | 0 (0) |
| 28. Isotretinoin in combination with a member of the tetracycline family of antibiotics | 0 (0) | 0 (0) |
| 29. Combined use of an oral and a local antibiotic | 0 (0) | 0 (0) |
| 30. Androgenic progestins (e.g., levonorgestrel, norgestrel, norethisterone, lynestrenol, dienogest, contraceptive implants, or vaginal rings) | 0 (0) | 0 (0) |
| 31. Use of aerosols for infants, children with asthma, or children showing asthma-like symptoms, such as dyspnea | 0 (0) | 0 (0) |
| 32. Combination of locally applied and orally administered antibiotics | 0 (0) | 0 (0) |
| 33. Topical agents containing corticosteroids | 0 (0) | 0 (0) |
| 34. Topical agents containing acyclovir before 6 years of age | 1(0.05) | 0 (0) |
| 35. A strong dermocorticoid (clobetasol propionate, with 0.05% dermoval, and betamethasone dipropionate, with diprosone) applied to the face, armpits or groin, and the backside of babies or young children | 8 (0.39) | 0 (0) |
| 36. Topically applied 0.03% tacrolimus before 2 years of age | 0 (0) | 0 (0) |
| 37. Topically applied 0.1% tacrolimus before 16 years of age | 0 (0) | 0 (0) |
| 38. Tricyclic antidepressants to treat depression | 14 (0.69) | 0 (0) |
| 39. Desmopressin administered by a nasal spray | 11 (0.54) | 0 (0) |
| 40. Tricyclic agents in combination with anticholinergic agents | 0 (0) | 0 (0) |
| 41. Tricyclic agents as a first-line treatment | 0 (0) | 0 (0) |
| 42. Cyproheptadine and clonidine | 6 (0.29) | 44 (0.62) |
| 43. Pharmacological treatment (attention deficit hyperactivity disorder) before 5 years of age (before school), except in severe cases | 6 (0.29) | 0 (0) |
Abbreviation: NSAIDs, non-steroidal anti-inflammatory drugs.
a Values are expressed as No. (%).
Because the adult cold contains acetaminophen, then it is regarded as acetaminophen. Prescribing drugs other than acetaminophen as the first line of treatment is a major IP in Mashhad. Nevertheless, in Tabriz, the highest error occurred in using antitussives before 2 years of age. Furthermore, the combined use of two antipyretics for pain relief in Tabriz occurred at a high rate. Ibuprofen was the most popular analgesic for fever and pain-related prescriptions in Mashhad. Acetaminophen and ibuprofen were the most often prescribed medications miswritten, according to the concurrent administration of both antipyretics and analgesics. Moreover, one of the most typical IPs was the first-line use of two non-steroidal anti-inflammatory drugs (NSAIDs). Ibuprofen, diclofenac, and piroxicam were administered together.
Metoclopramide, domperidone, ondansetron, and oral rehydration salt (ORS) powder were the primary medications used by physicians to manage gastrointestinal problems, diarrhea, nausea, and vomiting (Table 3). Pediatricians are not permitted to use metoclopramide as an antiemetic. As indicated in Table 3, metoclopramide was recommended in 81 and 132 patients in Mashhad and Tabriz, respectively. Additionally, it was found that Mashhad had the most remarkable rate of improper prescriptions for metoclopramide in its injectable form.
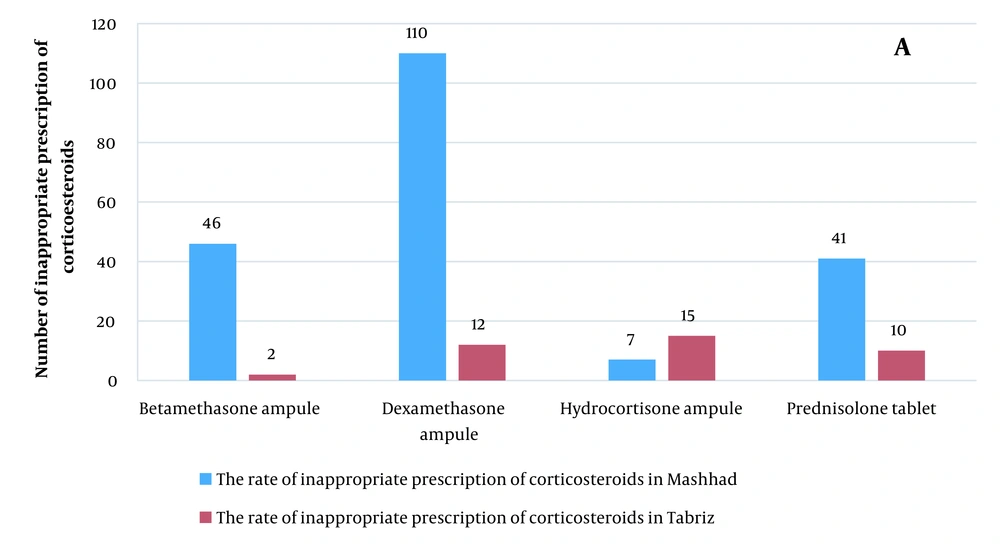
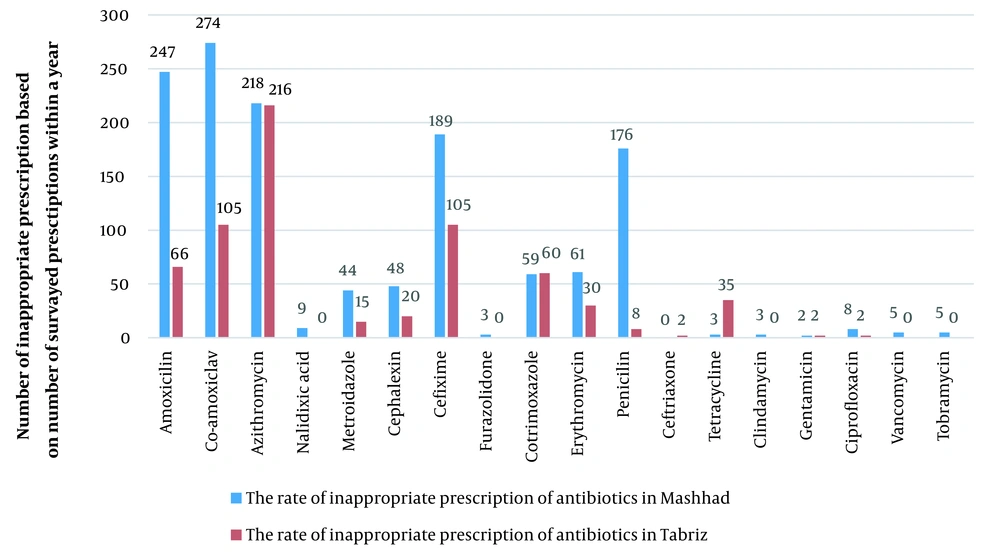
Most corticosteroid medications were written for the injectable form of hydrocortisone and dexamethasone in Tabriz and injectable form of betamethasone and dexamethasone in Mashhad (Figure 1). Antibiotics were frequently prescribed in the prescriptions studied in Mashhad (n = 1354 (66%)) and Tabriz (n = 666 (9.4%)). Co-amoxiclav, amoxicillin, azithromycin, cefixime, and penicillin were the most often recommended antibiotics in Mashhad; however, azithromycin, co-amoxiclav, cefixime, amoxicillin, cotrimoxazole, and tetracycline were the most frequently prescribed antibiotics in Tabriz. Overall, co-amoxiclav, cefixime, azithromycin, and amoxicillin were usually prescribed in both Mashhad and Tabriz. Figure 2 depicts the number of prescriptions for some antibiotics.
The administration of antihistamines, decongestants, antitussives, and expectorants were identified in Mashhad and Tabriz. The most frequently prescribed antihistamine was ketotifen, which experts also believe is safe for children. However, first-generation antihistamines, particularly diphenhydramine, were prescribed much more frequently than second-generation antihistamines. The most commonly recommended antihistamines, after ketotifen and diphenhydramine, were loratadine and cetirizine, except for pediatric cold syrup, which was also used in other circumstances.
The present study showed that cold medicine had the most prescriptions in the treatment of bronchiolitis and inflammation of the respiratory tract. Sputum medications, expectorants, decongestants, and antitussives were in the following ranks: Based on POPI criteria, 7.3% of IPs are related to sputum medications, expectorants (4.28%) before the age of 2, and 2.4% are related to decongestants (Table 3).
5. Discussion
To the best of our knowledge, there are few studies that evaluated the applicability of the POPI tool and modified it for application to regional pediatric practice, and this is the first study in Iran. Therefore, the current study’s results are not comparable to the results of other studies.
There has not been enough research on pediatric rational medication prescription (19, 20). The POPI is the first instrument to detect negligent or improper prescriptions, particularly for children (13). The POPI criteria are designed based on the same classification system as the STOPP/START criteria (i.e., according to the primary biological system) for prescription medications to pediatric patients (21, 22). Despite the rarity of multi-drug prescriptions for children, not many healthcare providers consult with or write prescriptions for pediatric patients.
The POPI tool in this study was developed using the Delphi method and several of the main techniques used to design tools to identify IPs. Although some studies have examined medicinal errors (22, 23), not a single study has examined the relationship between the rate of medication errors and the rate of side effects in pediatric patients based on POPI standards. For the first time in Iran, a modified and comprehensive tool for IPs (POPI) in children was obtained in Mashhad and Tabriz.
In the current analysis, over a quarter (35% in Tabriz) or even more than half (69% in Mashhad) of the prescriptions had at least one inappropriate medicine. The prevalence of IPs detected by modified POPI in the current study is much higher than that detected by POPI in other countries (9, 17, 24). Various prevalence rates of improper prescriptions have been reported, possibly due to some factors, such as different research settings, age groups, and national guidelines.
The frequent use of a drug other than acetaminophen as the first line of therapy is most likely because NSAIDs are mainly free of side effects commonly associated with opioids (25) and control chronic pain associated with inflammatory disorders. The use of H1 antagonists in young children is typically discouraged due to the potential for drowsiness, dizziness, and incoordination in an overdose. Additionally, no evidence has been obtained to support the use of sedating antihistamines in treating the symptoms of common colds in children. Metoclopramide was commonly used in this study. However, it is not recommended in POPI tools as it tends to cause extrapyramidal side effects, tardive dyskinesia, and drowsiness, although research has shown that they are temporary and do not have long-term repercussions.
The use of antibiotics was common in the studied prescriptions. The antibiotics were not properly evaluated because the rationale for their use could not be ascertained and was not always documented on the prescription sheets. This study also reported the use of corticosteroids in children. Corticosteroids are the basis of treatment for several pediatric disorders, especially in the acute phase. However, they are increasingly being replaced due to the long list of side effects. Before recommending systemic corticosteroids, clinicians should carefully consider the advantages and disadvantages.
Unexpectedly, this updated POPI might be loaded on the prescriber’s system (Electronic Prescribing software) and enables the system users (i.e., doctors and pharmacists) to alter their behavior/practice through a computer alarm system, for instance, professional behavior, activity, or performance, such as proper prescription or adherence to clinical recommendations. It is expected that the widespread use of this adopted POPI would assist the medical profession in lowering prescription error rates and improving pediatric health. It is hoped that physicians will use these criteria more frequently to minimize the risk of errors and adverse effects to ensure patient health.
The present study had several strengths. To the best of our knowledge, this is the first study conducted in Iran modifying the POPI criteria based on the INF and current clinical guidelines to improve its applicability. Furthermore, these criteria have been tested in an actual clinical practice setting and validated.
One issue with the current study relates to the information gathered using a Delphi method. This information represents only the views of chosen experts about a precise practice at a particular time, and the outcomes might vary depending on the experts involved in the panel. Secondly, these criteria can only be used as a screening tool for potential IPs and cannot directly determine the final rationality of prescriptions in place of comprehensive clinical assessment. Nearly all of the above medications can be used in specific conditions after the children’s overall clinical situation has been fully assessed. The present study did not include many drugs not supported by insurance companies because they are not always documented on the prescription sheets. Finally, the modified POPI was only intended to provide medication warnings to pediatric clinicians or pharmacists.
5.1. Conclusions
The modified POPI criteria are similar to those used in France but more localized and ready for use in Iran. Clinical validation and reliability studies in the usual care setting are needed and planned by the authors to evaluate the usability and reliability of this tool in routine practice.