Fulltext
Cardiofaciocutaneous syndrome (CFC) is a multiple congenital anomaly syndrome characterized by craniofacial features, cardiac defects, ectodermal anomalies and neurocognitive delay[1]. CFC is caused by mutations in BRAF, MEK1, MEK2, KRAS genes encoding proteins of the RAS/MAPK signaling pathway. In more than 70% of CFC patients, BRAF mutations are detected[2].
We present here a 10-year-old boy who was referred to the department of Medical Genetics for dysmorphological evaluation because of severe developmental delay, short stature and dysmorphic features. He was the third child of healthy, non-consanguineous Turkish parents. His parents and two siblings were healthy. He was born at term after an uneventful pregnancy. His birth weight was 3000 g (10-25th centile), height 50 cm (50th centile). His developmental milestones were globally delayed.
At the age of 10, his height was 87.5 cm (<3rd centile), his weight 15300 g (10-25 centile) and head circumference 49,5 cm (<3rd centile). Physical examination revealed coarse facial appearance, low-set ears, sparse eyebrows, bilateral ptosis, down-slanted palpebral fissures, epicanthal folds, bulbous nose, prominent philtrum, high-arched palate, thick lower lip, pectus excavatum, clinodactyly of fifth fingers (Fig. 1a). His hair was curly. Two cafe-au-lait spots were also noted. A hypertrophic cardio-myopathy was detected by echocardiography. On neurological examination, he had severe mental retardation (IQ below 50) with poor social interaction at the age of 10. Myopia was detected on ophthalmical examination. Fundus examination and visual evoked potentials were normal. Laboratory tests were normal. Magnetic resonance imaging showed cortical atrophy of the brain. Abdominal ultrasonography, X-ray of vertebral column and extremities were normal. A hearing test was normal. His karyotype was 46 XY.
On the basis of the observed facial dysmorphisms, hypertrophic cardiomyopathy and mental retardation, he was diagnosed as CFC. Genomic DNA from blood lymphocytes of the patient was isolated. Seven coding exons (6, 11-16) in BRAF were amplified by polymerase chain reaction. The polymerase chain reaction products were gel-purified and sequenced on an ABI PRISM 310 automated DNA sequencer (Applied Biosystems). A novel, missense heterozygous c.1442C>A mutation in exon 12 was identified in the proband. This mutation was not detected in both parents (Fig. 1b).
The cardio-facio-cutaneous syndrome (CFC) was first reported by Reynolds et al in 1986[3]. Overlapping phenotypes of patients with CFC, Costello Syndrome (CS) and Noonan Syndrome (NS) have been recognized [4]. Digilio et al
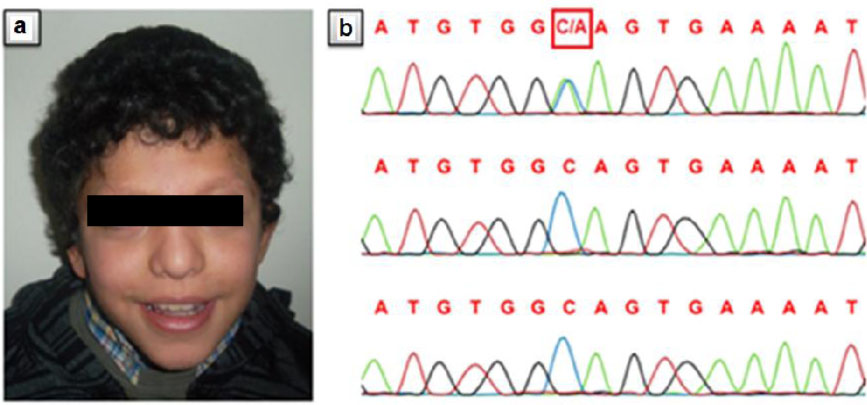
Fig. 1: Clinical features of the proband and sequencing chromatogram A: The patient at the age of ten years. Note curly hair, sparse eyebrows, down-slanted palpebral fissures, epicanthal folds, bulbous nose, prominent philtrum, thick lower lip. B: Chromatogram of the heterozygous change, c.1442C>A, in the proband (upper trace). Both parents (middle and bottom trace) are wild type at this position.
reviewed the literature and reported that while a depressed nasal root, curly-sparse hair were present more often in patients with CFC and CS than in patients with NS[5], the frequencies of low set ears were similar in these syndromes. Ptosis is more commonly seen in patients with CFC and NS than in patients with CS. Sparse or absent eyebrows have been reported in CFC and CS. Cutaneous features including loose skin on the hands and feet, deep palmoplantar creases, and perinasal papillomata is specifically associated with CS[5]. The craniofacial features of our patient, including curly hair, low-set ears, sparse eyebrows, bilateral ptosis, down-slanted palpebral fissures, epicanthal folds, depressed nasal root and bulbous nose are consistent with CFC.
The frequency of postnatal growth retardation in CS, CFC and NS have been reported as 97%, 67% and 30-50%, respectively[5]. Neurological abnormalities including hypotonia, motor and speech delay and learning disabilities are typically present in CFC and CS, which distinguishes these syndromes from NS[5]. Developmental delay of variable degree have been reported in 100% of patients with CS and in 93% of patients with CFC[5]. However, in 70-75% of patients with NS development is normal[4]. Our patient had short stature and severe motor and mental retardation with poor social interaction.
Cardiac abnormalities have been reported in CFC, CS and NS[4]. Most common cardiac abnormalities were pulmonic stenosis (69.7%), atrial septal defects (36.4%) and cardiomyo-pathy (21.2%) in CFC[1]. A hypertrophic cardiomyopathy was detected in our patient as cardiac abnormality.
CFC is a heterogeneous disorder that can be caused by mutations in BRAF, KRAS, MEK1 and MEK2[1,4]. In more than 70% of the patients, mutations are present in BRAF which is located at 7q34 and is composed of 18 exons spanning a region of 190 Mb[4]. The authors recommend that the sequence analysis of the seven BRAF exons (exons 6,11-16) is the first step for genetic diagnosis of CFC because of the majority of mutations are seen in these regions[4]. We detected a novel missense mutation c.1442C>A in exon 12 by sequence analysis of suggested exons. This mutation causes an A481E aminoacid change and it is found in the kinase domain which is located in the CR3 domain of BRAF. Regarding the findings found in our patient, this novel mutation probably causes severe motor mental retardation and classical dysmorphological features of CFC.
As a result, the patients who are considered to have CFC should be carefully examined for differential diagnosis of similar RASopathies. We also would like to emphasize that the sequence analysis of the seven BRAF exons should be done in the first step of molecular diagnosis of CFC.