1. Background
One of the most prevalent chronic disorders in children, with an estimated prevalence of 3% worldwide, is constipation which causes a significant health problem (1, 2). About 40% of children experience constipation in the first year of life (3), and about half of them also have fecal incontinence (4). Physical and psychological health problems may increase if constipation continues into adulthood (5). For children with constipation, the cost of health services per year is three times higher than that for children without constipation (6).
The recommended treatment includes dietary modification, behavioral reform, and using laxatives to assure that bowel movement occurs normally (7). Previous studies showed that polyethylene glycol could be used as a laxative in children. Polyethylene glycol is an inert polymer, soluble, and nonabsorbable laxative, which acts by osmosis and volume expansion in the large intestine (8). Also, numerous studies have shown the effectiveness of polyethylene glycol as a treatment for chronic functional constipation (9-14). Dietary changes, including the addition of carbohydrates and fiber, could be beneficial besides medications (15, 16). Dietary fibers with high water-binding capacity affect gastrointestinal motility by enhancing bacterial growth, their end-products, and feces volume, which improves colonic propulsion and facilitates defecation (7, 17).
The seed husk of Plantago ovata (psyllium husk), used as a prebiotic in partial hydrolysis form (18), can be investigated for its effectiveness in chronic constipation (19). Psyllium contains active compounds such as tannins, 4-omethylglucuronic acid, linoleic acid, oleic acid, palmitic acid, aucubin, campesterol, L-asparagine, L-cystine, mucilage, rhamnose, sterol, b-sitosterol, polysaccharides, and arabinoxylans (20).
Probiotics can be used to help prevent and treat diarrhea and constipation (21, 22). They can adjust the intraluminal region by enhancing the bacterial fermentation of final products, which affects the capacity of secretion and absorption of electrolytes, and decreases intraluminal pH (23).
Previous studies evaluated the efficiency of probiotics as a treatment for constipation (21, 24-29). It has been demonstrated that probiotics adjust the frequency of bowel movements and soften stools in adults with functional constipation. Although an uncontrolled pilot study in children suggested the possibility of benefit, the bulk of the available evidence failed to show any significant impact on objective measures of constipation (21, 24, 25, 28). Functional constipation in childhood may affect the quality of life in adulthood.
2. Objectives
This study aimed to compare the effects of psyllium seed husk powder versus polyethylene glycol with and without probiotics on constipation in children.
3. Methods
3.1. Subjects
The population of this study included all children in the age range of 2 - 12 years referred to the outpatient clinic of Shahid Beheshti Hospital in Kashan, Iran, from 2019 to 2020 due to acute functional constipation. The initial diagnosis of functional constipation was according to the Rome IV criteria (30) as follows: Having two or fewer defecations per week; history of excessive stool retention; history of painful or hard bowel movements; history of large-diameter stools that may obstruct the toilet; presence of a large fecal mass in the rectum; and at least one episode/week of incontinence after the acquisition of toileting skills.
The exclusion criteria were existence of gastrointestinal danger symptoms; recent treatment of constipation; gastrointestinal surgeries; having diabetes; existence of gastrointestinal anomalies; any organic causes for constipation; taking other medications that cause diarrhea or constipation; and psychological and behavioral disorders such as mental retardation and autism spectrum disorders.
3.2. Study Design
All the parents of included participants signed an informed consent prior to study. All parents received a coded package and explanation on how to use the medication. Dietary advice and toilet training were also provided to all parents. A questionnaire including demographic information and daily disease progression was filled for each patient by a pediatric assistant. Then, during the daily visits, the patient’s status in terms of duration of constipation and number of bowel movements per week was recorded in the questionnaire.
Since four methods were used to treat constipation, the patient or his/her family was informed about the group in written or by phone to keep people from mentioning the results. In addition, the results were communicated to the patient at the next visit. Using the simple randomization method, the patients were randomly divided into four equal groups (n = 36 each). Table 1 describes the treatment for each treatment group.
| Treatment Group | Number of Patients in Each Group | Treatment |
|---|---|---|
| A | 36 | Receiving 6 g per day of polyethylene glycol |
| B | 36 | Receiving 6 g per day of polyethylene glycol with probiotics |
| C | 36 | Receiving 6 g per day of psyllium |
| D | 36 | Receiving 6 g per day of psyllium with probiotics |
To compare the quantitative variable in two or more groups, the sample size was calculated using the following formula:
Where µ, mean of the outcome variable in groups 1 and 2; δ, standard deviation of the outcome variable in groups 1 and 2.
The calculation was done for all study groups separately, and the highest sample size was selected for the study. Considering type 1 error equal to 5%, statistical power of 90%, and effect size of 0.5, the minimum sample size in each group was calculated as 36 people. The number for bowel movements and painless bowel movements were checked in all patients after three weeks.
Psyllium and polyethylene glycol were purchased from Tak Zhen Zist Co, Iran. The raw materials, including psyllium and polyethylene glycol were formulated with a certain amount of probiotic in the Food Chemistry Laboratory of Kashan University of Medical Sciences, Iran daily and packed as a sachet form. Each sachet contained 6 g of laxative (polyethylene glycol or psyllium) with 109 CFU bacterial probiotics, including Lactobacillus reuteri, Lactobacillus rhamnosus, and Bifidobacterium infantis. Meanwhile, the same sachets without probiotics were prepared. The bacteria were mixed with equal proportions. Then, 109 CFU of bacteria were added to the psyllium packages, and the same number of probiotics was added to the polyethylene glycol packages.
To prepare the drug and make it double-blind, the drugs were prepared in identical packages and unlabeled sachets with only codes, and the total sachets were placed in a box of 15 sachets. The patient and the researcher were not aware of the contents of each box.
3.3. Analysis
The Kolmogorov-Smirnov test was used to evaluate the normality of the data. Paired t-test was applied to analyze the data. A 95% confidence level was considered as statistically significant. The baseline characteristics were statistically analyzed by using SPSS software (version 16.0.1, SPSS Inc.).
4. Results
The sex and mean values of age in children with functional constipation in different treatment groups are presented in Table 2. An independent t-test demonstrated no significant difference between the mean of sex (P = 0.74) and age (P = 0.45).
| Parameters | Treatment Group | P-Value | |||
|---|---|---|---|---|---|
| A | B | C | D | ||
| Sex | 0.74 | ||||
| Male | 20 | 18 | 16 | 16 | |
| Female | 16 | 15 | 20 | 20 | |
| Age (y) | 4.03 ± 2.32 | 5.56 ± 2.65 | 5.78 ± 2.59 | 5.42 ± 2.24 | 0.45 |
| Duration of constipation before treatment | 3.42 ± 0.649 | 3.58 ± 0.554 | 3.67 ± 0.632 | 3.64 ± 0.683 | 0.639 |
| The average number of bowel movements per week after treatment | 6.58 ± 0.604 | 6.72 ± 0.513 | 5.17 ± 0.231 | 5.83 ± 1.028 | 0.001 |
| The average number of painless bowel movements per week after treatment | 6.08 ± 1.079 | 6.36 ± 0.683 | 4.50 ± 1.483 | 5.19 ± 1.261 | 0.001 |
a Data were expressed as mean values ± standard deviation.
b n = 36 for all treatment groups.
As Table 2 shows, there was no significant difference between the mean duration of constipation before treatment in different groups (P = 0.6). Also, the average number of defecations per week after treatment was statistically different in various treatment groups (P = 0.001). The highest value was registered for the polyethylene glycol group with probiotics, and the lowest was observed in the patients treated with psyllium alone.
The results of the average number of painless bowel movements after three weeks of intervention are presented in Table 2. As can be seen, the effect of various treatments was significant on the average number of painless bowel movements (P = 0.001). The highest and lowest values for the average number of bowel movements and painless bowel movements were observed for the B and C groups, respectively.
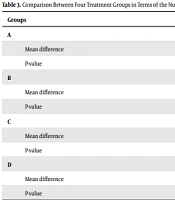
Table 3 presents a comparison between the four treatment groups in terms of the number of bowel movements after treatment. As can be seen, there was no significant difference between the two groups treated with polyethylene glycol (P = 0.9), but there was a statically significant difference between the psyllium group with and without probiotics and the polyethylene glycol group (P = 0.001). There was also a significant difference between the group treated with psyllium and the group treated with psyllium with probiotics (P = 0.018).
| Groups | A | B | C | D |
|---|---|---|---|---|
| A | ||||
| Mean difference | -0.1 | 1.4 | 0.7 | |
| P-value | 0.9 | 0.001 | 0.006 | |
| B | ||||
| Mean difference | 0.1 | 1.5 | 0.8 | |
| P-value | 0.9 | 0.001 | 0.001 | |
| C | ||||
| Mean difference | -1.4 | -1.5 | -0.6 | |
| P-value | 0.001 | 0.001 | 0.018 | |
| D | ||||
| Mean difference | -0.7 | -0.8 | 0.6 | |
| P-value | 0.006 | 0.001 | 0.018 |
Table 4 compares the four groups in terms of the number of painless bowel movements after treatment. As can be seen, there was no significant difference between the two groups treated with polyethylene glycol (P = 0.7), but there was a statically significant difference between the group treated with psyllium with and without probiotics and the groups treated with polyethylene glycol (P = 0.001).
| Groups | A | B | C | D |
|---|---|---|---|---|
| A | ||||
| Mean difference | -0.2 | 1.5 | 0.8 | |
| P-value | 0.7 | 0.001 | 0.008 | |
| B | ||||
| Mean difference | 0.2 | 1.8 | 1.1 | |
| P-value | 0.7 | 0.001 | 0.001 | |
| C | ||||
| Mean difference | -1.5 | -1.8 | -0.6 | |
| P-value | 0.001 | 0.001 | 0.06 | |
| D | ||||
| Mean difference | -0.8 | -1.1 | 0.6 | |
| P-value | 0.008 | 0.001 | 0.06 |
5. Discussion
According to our results, the effect of probiotic and polyethylene glycol on treating constipation in children was considerable. Also, polyethylene glycol was associated with a significantly higher effect than psyllium. Similar success was observed for the probiotic in combination with treatments.
The obtained data showed that children in both treatment groups of polyethylene glycol with and without probiotics defecated at least once every day, that is a normal defecation rate in children. A lower defecation rate was observed for treatment groups of psyllium with and without probiotics. Despite the defecation frequency, the consumption of probiotics led to an increase in the rate of daily defecation. Although the frequency of defecation in the group treated with polyethylene glycol with probiotics was higher than in the group treated with polyethylene glycol, this difference was not statistically significant.
Favretto et al. reported that consumption of 30 mg/day of enriched cheese with Bifidobacterium lactis had a beneficial effect on the symptoms of constipation (31). In this study, enrichment of psyllium with probiotics improved the number of bowel movements in children, but the effect of enrichment with polyethylene glycol with and without probiotics was not significantly different with the polyethylene glycol group. Tabbers et al. reported no evidence for the effect of probiotics, symbiotics, and behavioral changes on the improvement of constipation. They concluded that high-quality clinical trial studies with good design are needed in the field of non-pharmacological treatment of constipation in children (32).
Although, in some studies, the results were significant, the clinical effect of probiotics was normal. This study showed that fortification of psyllium with probiotics improved the constipation symptoms, and adding probiotics to psyllium had a positive effect on treatment. Ojetti et al. evaluated the effect of Lactobacillus reuteri supplementation on functional constipation. They reported that using Lactobacillus reuteri supplementation for four weeks had positive results, and long-term use of this species could have beneficial effects on constipation (33). Similar results were obtained in the present study on the improvement of constipation symptoms by adding probiotics to psyllium.
5.1. Conclusions
In general, polyethylene glycol was more effective than psyllium seed husk powder on the symptoms of constipation in children. While adding probiotics to polyethylene glycol did not significantly improve the symptoms, adding probiotics to psyllium had a positive effect on improving the symptoms and increasing the number of bowel movements.