1. Background
Eosinophilic esophagitis (EoE) is a chronic esophageal disease caused by immune system reaction and is clinically characterized by the symptoms of esophageal dysfunction and histologically characterized by an inflammatory process in which eosinophils dominate (1, 2). The EoE is caused by an abnormal immunologic response to specific antigens. Food antigens are the most common allergens responsible for EoE, and respiratory antigens are in second place (3). The prevalence of the disease is rising (4, 5), and EoE is the second most common cause of chronic esophagitis (6). The disease could occur at any age. Almost 75% of the patients are male, and the mean age of children is 8 - 10 years (7). Up to 50% of patients are affected by other allergic diseases, such as asthma, eczema, or allergic rhinitis (8). The clinical manifestations of EoE differ by age. Among adults and teens, the disease may emerge as dysphagia and food impaction. In younger children, it represents eating disorders which could be abnormal eating patterns (e.g., only drinking liquids or eating soft foods), gaining adaptive habits (e.g., refusing solid foods which have already been eaten, eating slowly, over-chewing, over-drinking liquids with meals), failure to thrive or reflux symptoms (e.g., vomit, regurgitation, water brash, epigastric pain, heartburn, and chest pain) (9).
In patients with esophageal dysfunction and endoscopic findings in favor of EoE who have eosinophil predominance in the biopsy, EoE is confirmed. Mucosal eosinophilia must be observed without other causes, such as infections and medications (10, 11). EoE treatment is based on identifying and eliminating dietary antigens, while topical steroids given by metered dose inhaler (MDI) or swallowing a viscous solution are effective therapeutic options (12). Short-course systemic steroids and biologic treatments, such as Reslizumab and Mepolizumab, are rarely used (13).
A comprehensive literature review showed that in infants and children, unlike adults, ultrasound has a high sensitivity of 87% and an average specificity of 63% compared to the gold standard of a 24-hour pH-monitoring test for GERD diagnosis (14). Unfortunately, the only definite approach currently available to evaluate disease activity is endoscopy with biopsy, and it is necessary to perform the procedure several times for each patient, which would cause costs and potential complications (15). This study investigated the possibility of replacing a non-invasive method with standard invasive methods, such as endoscopy.
2. Objectives
We evaluated esophageal ultrasound parameters in children with a definite diagnosis of EoE and GERD as well as healthy children to find out whether there are specific ultrasound findings for EoE patients to differentiate them from GERD and healthy children. In addition, we assessed the possibility of replacing ultrasound with an invasive endoscopic method for the diagnosis and follow-up of EoE.
3. Methods
3.1. Study Setting
This cross-sectional study was conducted in 2021 on children referred to the Isfahan (Pediatric Gastroenterology Clinic of Teaching and Referral Hospital in Isfahan).
3.2. Participants
A total of 90 children were enrolled in three groups of equal numbers. The participants were in the age range of 4 - 12 years. The inclusion criteria for the control group were healthy children with normal growth and development and no digestive symptoms who visited for routine care. The GERD group were children with GERD whose signs were consistent with GERD and had no optimal response to treatment or had recurrence when the treatment stopped. These cases underwent endoscopy and biopsy to rule out other differential diagnoses, such as EoE, and had endoscopic and pathologic findings consistent with GERD. The third group had a definite diagnosis of EoE based on clinical symptoms and had a poor response to an 8-week course of proton pump inhibitor medication prior to endoscopy. They had endoscopic evidence of EoE and more than 15 eosinophils per high-power field in the esophagus with no eosinophilia in other gastrointestinal tract sites. The exclusion criteria were the recognition of obstructive disease of the gastrointestinal tract other than EoE or metabolic or systemic disease, taking corticosteroids, and low compliance for ultrasound assessment.
3.3. Ethical Considerations
After explaining the research protocol, oral assent was obtained from children, and written informed consent from their parents. Conventional ultrasound was a non-invasive procedure. The families did not pay for the costs and could leave the study anytime they intended to. The data were provided anonymously. The study was approved by the Ethics Committee of Isfahan University of Medical Sciences (IR.MUI.MED.REC.1398.218).
3.4. Methods
In these three groups, the ultrasound parameters, such as diameter, wall thickness, and distensibility of the cervical and abdominal esophagus, the mucosal thickness of the cervical esophagus, hiatal diameter, sub-diaphragmatic esophageal segment length, and gastric (cardia) wall thickness were determined by an experienced pediatric radiologist. The ultrasound system used for examining the esophagus was GE (model E8). The radiologist and data analyst of the study were uninformed of the grouping and diagnosis of patients.
3.5. Examination of Cervical Esophagus
The cervical esophageal examination of a patient was completed in a supine position with small cushions under his shoulders using a surficial probe (MHZ linear 7.5). The mucosal and wall thickness, as well as lumen diameter, were measured under the above conditions. Then, the patient was given about 10 - 20 cc of water to keep in his mouth. Next, he was supine again, and after placing the probe in the proper position, the patient was asked to swallow the water in his mouth so that the lumen diameter of the esophagus could be measured as the liquid passed through it (16).
3.6. Abdominal Esophagus Examination
The patient was in the supine position, and the procedure was performed utilizing a deep probe (MHZ curve 5). The probe was put in the xiphoid region in an oblique direction so that the length of the abdominal esophagus could be seen in the image field. Under the mentioned conditions, the lumen diameter, wall thickness, and length of the abdominal esophagus up to the hiatus region were measured. In addition, cardiac wall thickness and hiatus diameter were measured. Afterwards, about 10 - 20 cc of water was given to the patient to keep it in his mouth, he was supine again, and after placing the probe in the proper position, the patient was asked to swallow water in his mouth so that the esophageal lumen diameter could be measured as the liquid passed through it (16).
3.7. Statistical Methods
The obtained values for all three groups were compared so the differences in the ultrasound findings of EoE patients, GERD patients, and control children could be determined. Continuous variables were expressed as mean ± SD. In addition, the number (percentage) was used for categorical data. The means of subjects in groups EoE, GERD, and control were compared by one-way analysis of variance and Tukey’s post hoc test. The chi-square test was used to assess the difference in the distribution of categorical variables between EoE and GERD groups. When the chi-square test was not suitable due to the small sample size, Fisher's exact test was used. Receiver operating characteristic (ROC) curves were used to evaluate the general performance of ultrasound findings to discriminate EOE from controls and GERD, as well as GERD from controls. ROC analysis provides the area under the ROC curve (AUC) and the sensitivity and specificity measures. The optimal cut-off was calculated for AUC ≥ 0.7. All statistical analyses were performed using SPSS version 20.0 for Windows. P-value < 0.05 was considered statistically significant.
4. Results
The data of 90 patients (i.e., 30 in each group) were analyzed. The mean ± SD age of participants was 7.78 ± 2.78 years, with the age range of 4 - 12 years for all three groups. Overall, 53.3% of participants were boys. Table 1 shows the characteristics of participants in three groups. The EoE patients were the youngest, with a mean ± SD of 6.50 ± 2.35 years. The gender ratio and BMI of participants were not significantly different between the three groups (P > 0.05). Table 2 presents the comparison of ultrasound findings between the three groups. The differences in the mean abdominal esophageal wall thickness were significant between the three groups of control, EoE, and GERD (P = 0.002). The EoE group had the highest, and the control group had the lowest mean abdominal esophageal wall thickness.
| Total | Control | EOE | GERD | P-Value | |
|---|---|---|---|---|---|
| Age (y) | 7.78 ± 2.78 | 8.27 ± 2.49 | 6.50 ± 2.35 | 8.58 ± 3.06 | 0.006 |
| BMI (kg/m2) | 15.81 ± 2.99 | 16.04 ± 2.85 | 15.15 ± 3.20 | 16.30 ± 2.87 | 0.312 |
| Breastfeeding (mo) | 18.82 ± 6.81 | 20.00 ± 7.28 | 17.63 ± 6.20 | 0.180 | |
| Gender | 0.175 | ||||
| Female | 42 (46.7) | 17 (56.7) | 10 (33.3) | 15 (50.0) | |
| Male | 48 (53.3) | 13 (43.3) | 20 (66.7) | 15 (50.0) |
a Values are expressed as mean ± SD or No. (%).
| Control | EOE | GERD | P | Peg | Pce | Pcg | |
|---|---|---|---|---|---|---|---|
| Abdominal esophagus | |||||||
| Diameter (mm) | 8.76 ± 1.55 | 8.76 ± 1.01 | 8.90 ± 2.19 | 0.950 | 0.985 | 1.000 | 0.990 |
| Wall thickness (mm) | 2.08 ± 0.51 | 2.73 ± 0.66 | 2.59 ± 0.93 | 0.002 | 0.733 | 0.002 | 0.020 |
| Length (mm) | 21.00 ± 6.00 | 21.30 ± 6.20 | 17.88 ± 5.45 | 0.050 | 0.069 | 0.979 | 0.106 |
| Gastric wall thickness | 3.24 ± 0.71 | 4.30 ± 0.79 | 3.34 ± 1.06 | < 0.001 | < 0.001 | < 0.001 | 0.896 |
| Percentage of distensibility changes after drinking water | 59.35 ± 25.32 | 44.70 ± 28.39 | 43.29 ± 25.24 | 0.037 | 0.977 | 0.086 | 0.053 |
| Cervical esophagus | |||||||
| Diameter (mm) | 7.00 ± 1.15 | 6.61 ± 1.19 | 5.74 ± 1.26 | < 0.001 | 0.016 | 0.435 | < 0.001 |
| Wall thickness (mm) | 1.65 ± 0.44 | 2.32 ± 1.21 | 1.75 ± 0.35 | 0.003 | 0.016 | 0.004 | 0.871 |
| Mucosal thickness (mm) | 0.78 ± 0.81 | 0.98 ± 1.08 | 0.69 ± 0.15 | 0.316 | 0.985 | 1.000 | 0.990 |
| Percentage of distensibility changes after drinking water | 92.09 ± 45.70 | 84.23 ± 38.80 | 119.72 ± 53.08 | 0.017 | 0.015 | 0.858 | 0.101 |
| Hiatal diameter (mm) | 8.35 ± 1.66 | 8.56 ± 1.50 | 8.86 ± 0.96 | 0.368 | 0.677 | 0.837 | 0.338 |
a P, P-value for comparing the means of control; EoE; and GERD groups; Peg, P-value for comparing the means of EoE and GERD groups; Pce, P-value for comparing the means of control and EoE groups; Pcg, P-value for comparing the means of control and GERD groups.
Moreover, the differences in the mean abdominal esophageal wall thickness between the control and EoE groups were significant. The means of gastric wall thickness were significantly different between the three groups (P < 0.001). The subjects in the EoE group had the highest mean gastric wall thickness. Furthermore, the EoE group was significantly different from the control and GERD groups in terms of the mean gastric wall thickness (P < 0.001). Differences in mean cervical esophageal diameter between the three groups were significant (P < 0.001). The GERD group had significantly different mean cervical esophageal diameters from the control and EoE groups. The differences in the mean cervical esophageal wall thickness between the three groups were significant. The EoE group had the highest mean cervical esophageal wall thickness. Moreover, the differences of the EoE group with the GERD and control groups were significant. There were significant differences in the mean percentages of abdominal and cervical esophageal distensibility changes between the three groups (P < 0.05). The groups of control and GERD had the highest mean abdominal esophageal and cervical esophageal percentage of distensibility changes, respectively. Furthermore, the difference in the mean percentage of cervical esophageal distensibility changes between the EoE and GERD groups was significant (P = 0.017).
Tables 3, 4, and 5 show the results of the ROC curve analysis, including AUC, 95% confidence interval, P-value, the optimal cut-off for AUC > 0.7, as well as the sensitivity and specificity corresponding to their cut-off. The highest AUCs for discriminating EoE from controls were 0.832 (95% CI: 0.726 - 0.937) and 0.802 (95% CI: 0.690 - 0.914) for abdominal gastric wall thickness and abdominal esophageal wall thickness, respectively. Furthermore, abdominal esophageal wall thickness had the highest AUC of 0.706 (95% CI: 0.572 - 0.839) for discriminating GERD from controls. In addition, the highest AUCs for discriminating EoE from GERD were 0.800 (95% CI: 0.680 - 0.920) and 0.713 (95% CI: 0.578 - 0.848) for gastric wall thickness and cervical wall thickness, respectively.
| AUC (95 % CI) | P-Value | Optimal Cut Off | Se (%) | Sp (%) | PPV | NPV | |
|---|---|---|---|---|---|---|---|
| Abdominal esophageal course | |||||||
| Diameter | 0.549 (0.400 - 0.697) | 0.515 | |||||
| Wall thickness | 0.610 (0.462 - 0.758) | 0.143 | |||||
| Length | 0.659 (0.519 - 0.798) | 0.035 | |||||
| Gastric wall thickness | 0.800 (0.680 - 0.920) | < 0.001 | 2.95 | 100 | 60 | 76 | 74 |
| Percentage of distensibility changes after drinking water | 0.439 (0.292 - 0.587) | 0.420 | |||||
| Cervical esophageal course | |||||||
| Diameter | 0.702 (0.564 - 0.839) | 0.007 | 6.55 | 64 | 83 | 91 | 74 |
| Wall thickness | 0.713 (0.578 - 0.848) | 0.005 | 1.95 | 63.3 | 73 | 73 | 68 |
| Mucosal thickness | 0.681 (0.543 - 0.818) | 0.016 | |||||
| Percentage of distensibility changes after drinking water | 0.311 (0.167 - 0.455) | 0.013 | |||||
| Hiatal diameter | 0.433 (0.283 - 0.582) | 0.371 |
| AUC (95 % CI) | P-Value | Optimal Cut Off | Se (%) | Sp (%) | PPV | NPV | |
|---|---|---|---|---|---|---|---|
| Abdominal esophageal course | |||||||
| Diameter | 0.489 (0.338 - 0.641) | 0.888 | |||||
| Wall thickness | 0.706 (0.572 - 0.839) | 0.006 | 2.05 | 83.3 | 60 | 68 | 78 |
| Length | 0.351 (0.211 - 0.490) | 0.047 | |||||
| Gastric wall thickness | 0.468 (0.314 - 0.623) | 0.673 | |||||
| Percentage of distensibility changes after drinking water | 0.318 (0.181 - 0.456) | 0.016 | |||||
| Cervical esophageal course | |||||||
| Diameter | 0.223 (0.103 - 0.344) | < 0.001 | |||||
| Wall thickness | 0.562 (0.414 - 0.710) | 0.408 | |||||
| Mucosal thickness | 0.599 (0.454 - 0.744) | 0.188 | |||||
| Percentage of distensibility changes after drinking water | 0.665 (0.521 - 0.809) | 0.028 | |||||
| Hiatal diameter | 0.581 (0.430 - 0.731) | 0.284 |
| AUC (95 % CI) | P-Value | Optimal Cut Off | Se (%) | Sp (%) | PPV | NPV | |
|---|---|---|---|---|---|---|---|
| Abdominal esophageal course | |||||||
| Diameter | 0.549 (0.400 - 0.697) | 0.515 | |||||
| Wall thickness | 0.610 (0.462 - 0.758) | 0.143 | |||||
| Length | 0.659 (0.519 - 0.798) | 0.035 | |||||
| Gastric wall thickness | 0.800 (0.680 - 0.920) | < 0.001 | 2.95 | 100 | 60 | 71 | 100 |
| Percentage of distensibility changes after drinking water | 0.439 (0.292 - 0.587) | 0.420 | |||||
| Cervical esophageal course | |||||||
| Diameter | 0.702 (0.564 - 0.839) | 0.007 | 6.55 | 64 | 83 | 79 | 69 |
| Wall thickness | 0.713 (0.578 - 0.848) | 0.005 | 1.95 | 63.3 | 73 | 70 | 67 |
| Mucosal thickness | 0.681 (0.543 - 0.818) | 0.016 | |||||
| Percentage of distensibility changes after drinking water | 0.311 (0.167 - 0.455) | 0.013 | |||||
| Hiatal diameter | 0.433 (0.283 - 0.582) | 0.371 |
Abbreviations: Se, sensitivity; Sp, specificity; PPV, positive predictive value; NPV, negative predictive value.
a Optimal cut-offs were calculated using the maximum Youden index = (Se+Sp-1).
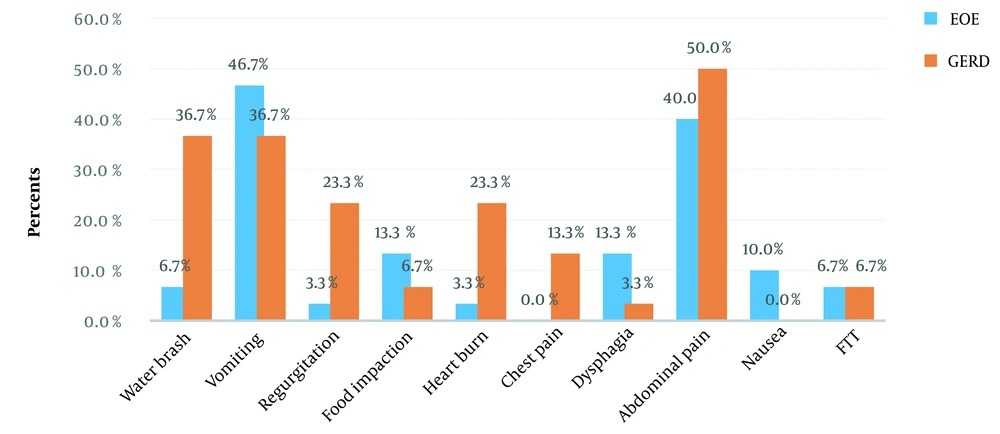
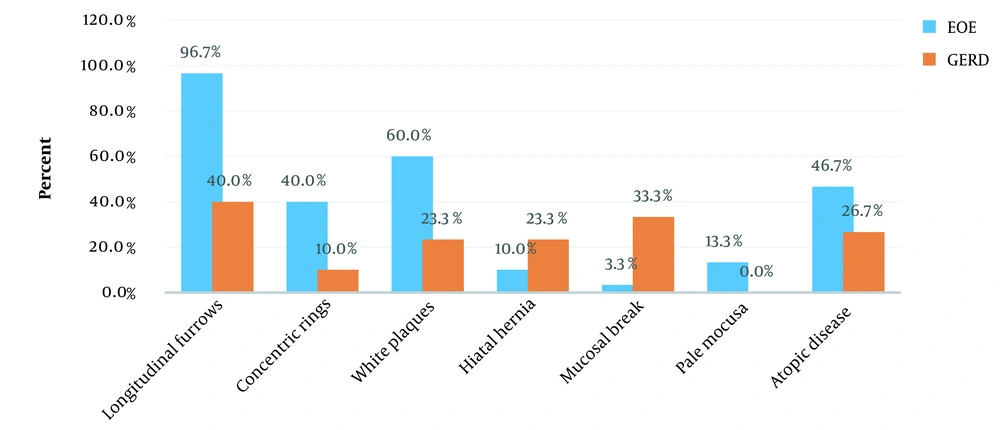
Figures 1 and 2 present the clinical symptoms and endoscopic findings of EoE and GERD. The frequency (percentage) of water brash, regurgitation, and heartburn for GERD was significantly higher than EoE (P < 0.05). Furthermore, the frequency (percentage) of longitudinal furrows, concentric rings, and white plaques for EoE were significantly higher than GERD (P < 0.05). The frequency (percentage) of a mucosal break for GERD was significantly higher than EoE.
5. Discussion
This study showed that although the means of EoE ultrasound findings were significantly different from the control and GERD groups, the ability of ultrasound findings to discriminate EoE from control and GERD groups was moderate. Therefore, ultrasound had only moderate value for diagnosing and following children with EoE and GERD. Eosinophilic esophagitis is a chronic disease, and symptoms recur as the patient’s diet or treatment is discontinued (17). Delayed identification and treatment of the disease could cause the risk of esophageal stricture (18).
One of the major problems in the care of patients with EoE is the lack of reliable and non-invasive means for primary diagnosis and treatment monitoring. Currently, the only reliable method is endoscopy which is precise but invasive and should be performed several times for each patient to enable diet control and assess the improvement or recurrence of the conflict. In addition to being expensive, this technique requires sedation in children and imposes a risk of anesthesia. High-resolution impedance planimetry and high-resolution endoscopic ultrasound are other methods introduced to assess the severity of EoE but are still dependent on endoscopy (19).
About 10%-33% of children diagnosed with EoE have normal esophageal mucosa with common endoscopic findings of adults, such as esophageal rarely observed in children. Consequently, in children, the valuable features of adult endoscopy alone do not make a reliable diagnosis of EoE or assess disease activity, and adult-like scoring is less important in diagnosing or evaluating response to treatment (20, 21). Recent studies suggested that ultrasound can be used for examining esophageal diseases, such as GERD-caused esophagitis. However, contradictory findings have been obtained (22-24). If a non-invasive and reproducible method, such as ultrasound, can provide a new approach to the severity and activity of EoE in children, it would be an ideal method for the early diagnosis and follow-up of response to treatment.
A study to diagnose induced esophagitis by GERD showed a moderate association between sonographic and endoscopic findings with 45% sensitivity and 86% specificity, while esophageal AP diameter in hiatus and GEJ had no significant association with esophagitis (23). Research revealed that, unlike the thoracic esophagus, the proximal esophagus could easily be assessed by ultrasound, and although the length of the cervical esophagus increased with age, the mean thickness of the cervical esophagus remained constant throughout the age. It was concluded that conventional ultrasound could be easily used to assess proximal esophageal diseases in children (25). In this study, the evaluated indices, including diameter, wall thickness, length, and percentage of dispensability changes after drinking water, in the cervical and abdominal esophagus, were measured by ultrasound, and we evaluated the diagnostic value of ultrasound findings in children with EoE.
Evaluation by high-resolution endoscopic ultrasound revealed a significant difference between patients with EoE and healthy children in terms of the mean total wall thickness and combined mucosa and submucosa and muscularis propria of the esophagus. However, there was no significant difference in circular muscle (21). In the present study, the mean gastric wall thickness of the EoE group was significantly different from the control and GERD groups (P < 0.001). Differences between the mean cervical esophageal diameters in the three groups were significant (P < 0.001).
The findings of a study showed that the thickness of mucosa and sub-mucosa and total wall thickness of the middle and distal esophagus increase as a function of the age and height of children. There was an insignificant difference between recorded values for central and distal esophagus, and the obtained values for control patients could be used for determining esophageal disease among children (26). We found no significant difference between the three groups regarding mucosal thickness in the cervical esophagus and diameter and length in the abdominal esophagus. In previous studies, the percentage of esophageal dilatation after swallowing fluids has not been studied by ultrasound. However, the measurement of esophageal distensibility by high-resolution impedance planimetry shows that measuring esophagus distensibility in EoE patients could be a new means of assessing the patients’ response to treatment over time (20).
Another investigation performed by high-resolution impedance planimetry indicated that decreased esophageal dilatation was associated with an increased risk of food impaction, and the need for esophageal dilatation was demonstrated during a follow-up of 4-12 months (27). In our study, there was a significant difference between the mean percentage of changes in the abdominal and cervical esophagus expansion after drinking water in the three groups (P < 0.05). The control group and GERD had the highest mean percentage of distensibility after drinking water for the abdominal and cervical esophagus, and lower distensibility in the EoE group can be considered a differentiating criterion.
5.1. Conclusions
The findings of this study showed that the mean differences in ultrasound findings were significant between the three groups of EoE, GERD, and control. However, the ability of ultrasound findings to discriminate EoE from control and GERD groups was moderate. Therefore, ultrasound is moderately beneficial for diagnosing and following up on children with EoE and GERD.
5.2. Limitations
The first limitation was the size of the study sample. Second, in this study, the mean age of patients in the EoE group was significantly younger than the control and GERD groups despite the fact that the BMI of the three groups was not significantly different between the three groups.
5.3. Recommendations
Further studies with larger sample sizes and without age differences between study groups may provide more precise results. This method is suggested for monitoring EoE patients' response to intended treatments in the long run with acceptable sample size.