1. Background
Necrotizing enterocolitis (NEC) is an intestinal disease seen in newborns. It is the most common cause of intestinal emergencies in newborns and is much more common in preterm infants. The incidence is 0.5 - 2.2 per 1000 live births (1). Newborns with low birth weight are especially at higher risk. NEC is a disease with long hospital stays. Its mortality rate varies from 10 to 60% (2). NEC can occur due to many factors, mainly enteral nutrition, bowel ischemia, abnormal intestinal flora, and immature intestinal mucosa (3). Excessive free oxygen radical and endogenous cytokine generation triggers the activation of several inflammatory pathways, including platelet-activating factor (PAF), tumor necrosis α (TNF-α), and interleukin 6. These unregulated pathways have a role in the pathogenesis of NEC (4, 5).
Flavonoids are phenolic compounds commonly found in plants (6). Flavonoids have several characteristics and act as antioxidants, anticarcinogens, sedatives, anti-inflammatories, and microcirculatory regulators; they are also involved in protection against cardiovascular diseases (7, 8). Germs, fruits of bitter oranges, olives, and grapefruits are rich in flavonoids (9). Hesperidin (Hsd), a flavonoid glycoside, is abundant in the peel of citrus fruits (10). Hsd also exerts an antiapoptotic effect; thus, it can reduce free oxygen radicals and stimulate endogenous antioxidant defense systems (11). Diosmin, another flavonoid glycoside, has antiapoptotic antioxidant and anti-inflammatory properties (12). It has been shown that S 5682, containing 90% diosmin and 10% Hsd, scavenges oxygen radicals and acts as a dose-dependent inhibitor of hyperglycemia along with anti-inflammatory and anti-edematous effects (13). It has been used for the treatment of venous insufficiency, varices, and hemorrhoids as a venotonic drug and venous regulator for many years.
Broad-spectrum antibiotics (14), cessation of oral feeding, and surgical intervention (15) have been used to treat NEC. The use of several substances, such as colchicines (16), N-acetyl cysteine (17), alkaline phosphatase (18), and dexpanthenol (19), has been shown experimentally in clinical studies for NEC treatment. In our literature review, no study has been conducted on Hsd and diosmin use in NEC treatment.
2. Objectives
This study aimed to search the effectiveness of Hsd and diosmin, which have antioxidant, anti-inflammatory, and microcirculatory regulation properties in the treatment of NEC, where oxidative damage and activation of inflammatory pathways and microcirculatory impairment play a crucial role in pathogenesis.
3. Methods
3.1. Animals and Experimental Design
This study was approved by the Experimental Animal Ethics Committee of Gaziosmanpaşa University, Medical School, and ethical rules of international animal experimentation were followed throughout the study (2012 HADYEK 27). Three equal groups of randomly selected newborn Sprague Dawley rats were prepared for this study (n = 10). Group I was the control group, group II constituted the NEC model group, and group III was the NEC model group that received flavonoid therapy.
3.2. NEC
Newborn rats in groups II and III were housed in an airtight plexiglass cage (Matrx, 145 Mid County Drive Orchard Park, USA). The NEC model was obtained in these rats by inhalation of 100% CO2 for 5 minutes. The rats were then given standard care. During the experiment, the animals were breastfed by their mothers (20).
Group I (the control group; n = 10) included rats that had not been exposed to hypoxia/ reoxygenation and had not undergone any treatment protocol. Rats in group II (without treatment; n = 10) were given distilled water in the same amount of flavonoid twice daily for 5 days after hypoxia/reoxygenation. In group III (flavonoid therapy; n = 10), 100 mg/kg of the flavonoid (Daflon 500, Servier) was administered by oral gavage twice daily after hypoxia/reoxygenation. On day 5 of the study, laparotomy was performed on the experimental animals following cervical dislocation under anesthesia. Tissue samples from the stomach, ileum, and cecum were obtained. These samples were then placed in a liquid nitrogen tank at -80 °C pending biochemical analysis. Malondialdehyde (MDA), superoxide dismutase (SOD), NO, and glutathione peroxidase (GSH-Px) levels were measured in these tissue samples. Other tissue samples were kept for histological examination to perform histopathological examination and immunohistochemical staining.
3.3. Biochemical Analysis
Oxidative stress markers were determined in gastrointestinal tissue homogenates by measuring MDA, SOD, and GSH-Px activities. The samples were prepared by hydrating them in a cold solution of 0.9% NaCl in a room at 22°C. Following the preparation, the samples were homogenized using a homogenizer (IKA Ultra-Turrax t 25, Germany), after which they were filtered and centrifuged. Supernatants were used to determine the enzymatic activities. The MDA level was measured using the method established by Esterbauer and Cheeseman (21). A maximum absorption peak of 532 nm was produced following the reactions, with the result being expressed in terms of grams of protein per nanomole (nmol/g protein). NO measurement readings were determined by the method described by Cortas and Wakid (22). The Griess reaction is based on nitrite and nitrate, and this method is considered an indication of NO production. Using a spectrophotometer at 545 nm, total nitrite (nitrite/nitrate) was measured, with the collected data expressed in terms of micromoles per gram of wet tissue (umol/g protein). The glutathione peroxidase (GSH-Px) activity was determined using the method established by Paglia and Valentine (23). This method starts the enzymatic reaction with the addition of H2O2, after which the change of absorption at 340 nm is assessed with the help of a spectrophotometer. Using the method described by Sun et al, the total (Cu/Zn and Mn) SOD activity was calculated (24). In this method, 1 unit of SOD is based on the reduction and inhibition of nitroblue tetrazolium (NBT), and the rate of NBT reduction is described as a load of enzymes resulting in 50% inhibition. Each GSH-Px and SOD were expressed as the same: units per gram protein (U/g protein)
3.4. Histopathological Study
Tissue samples taken from the stomach, ileum, and cecum were fixated in a 2% buffered neutral formaldehyde solution for 4 days. Afterward, the samples were placed within paraffin blocks and then cut using a rotary microtome (LEICA RM2125RT, China) with a thickness of 5 μm. The sections were kept for about 12 hours at 58 - 60°C to melt paraffin. Paraffin-melted sections were histologically stained with the triple staining protocol (modified Masson trichrome). Histological analyses were performed on an average of 7 - 10 sections per individual of each group and 50 different areas of each section. Areas of analysis were determined randomly using light microscopy with stereological software (Stereo Investigator; MBF Biosciences, Williston, USA). The analyses were performed as a blind study by 2 histologists encoding the preparations. To quantify the degree of histological damage, NEC histological injury scoring system described by Nadler et al. was used (25). The criteria in this scoring system are as follows: normal intestine (grade 0), enterocyte villi irregularity and mild villus core dissociation (grade 1), enterocyte villi irregularity and severe villus core dissociation (grade 2), desquamation of villus epithelium (grade 3), and intestinal necrosis/perforation (grade 4). The NEC damage scores of each animal were determined, and the mean score was calculated.
3.5. TUNEL Staining
Four to five micrometers-thick sections of paraffin-embedded gastrointestinal tissue specimens fixed with formalin were obtained. These sections taken from tissues in paraffin blocks were incubated, deparaffinized, and rehydrated according to standard kit applications (In Situ Cell Death Detection Kit, AP; Roche). The obtained materials were placed in 200 mL of 0.1 M citrate buffer in jars with a pH of 6. They were placed in a microwave oven and subjected to microwave irritation of 3.5 kW for 5 minutes. Afterward, the jar was cooled by adding 80 mL of distilled water at room temperature. The tissues were taken into phosphate-buffered saline (PBS) at room temperature. After soaking in Tris-HCl for about 30 minutes, they were submerged twice in PBS. Then, the excess liquid was removed. The sections were outlined using a hydrophobic pap pen (Liquid Blocker, Japan). Next, a TUNEL (terminal deoxynucleotidyl transferase–mediated biotin–deoxyuridine triphosphate nick-end labeling) reaction mixture of 50 µL (1:9; vial 1 + vial 2), which was prepared according to the standard kit protocol, was added to the mixture. The mixture was washed 3 times with PBS, after which the mixture was incubated for 60 minutes. FASTRED solution was used on the tissues, after which the tissues were further incubated for 5 minutes under a light microscope. Upon observing a specific color change, the reaction was terminated using PBS. The tissue sections were treated with a hematoxylin solution, and coverslips were closed with a water-based sealing solution (Aqueous-Mount, Invitrogen). Negative controls were performed using a light microscope by checking for the apoptotic indices of the preparations after dripping the TUNEL mixture on the preparations. There was no positive labeling in the negative control sections.
3.6. Statistical Analysis
Statistical analysis was performed using SPSS version 20 (SPSS Inc, Chicago, Ill, USA). Data were presented as mean ± SD. The homogeneity between the variables was examined by the Levene test. A 1-way analysis of variance (ANOVA) and Tukey test were used as a post-hoc test when comparing groups in terms of NEC score, oxidant-antioxidant levels, and apoptotic index. A P-value < 0.05 was considered statistically significant.
4. Results
4.1. Histopathological Injury Scoring
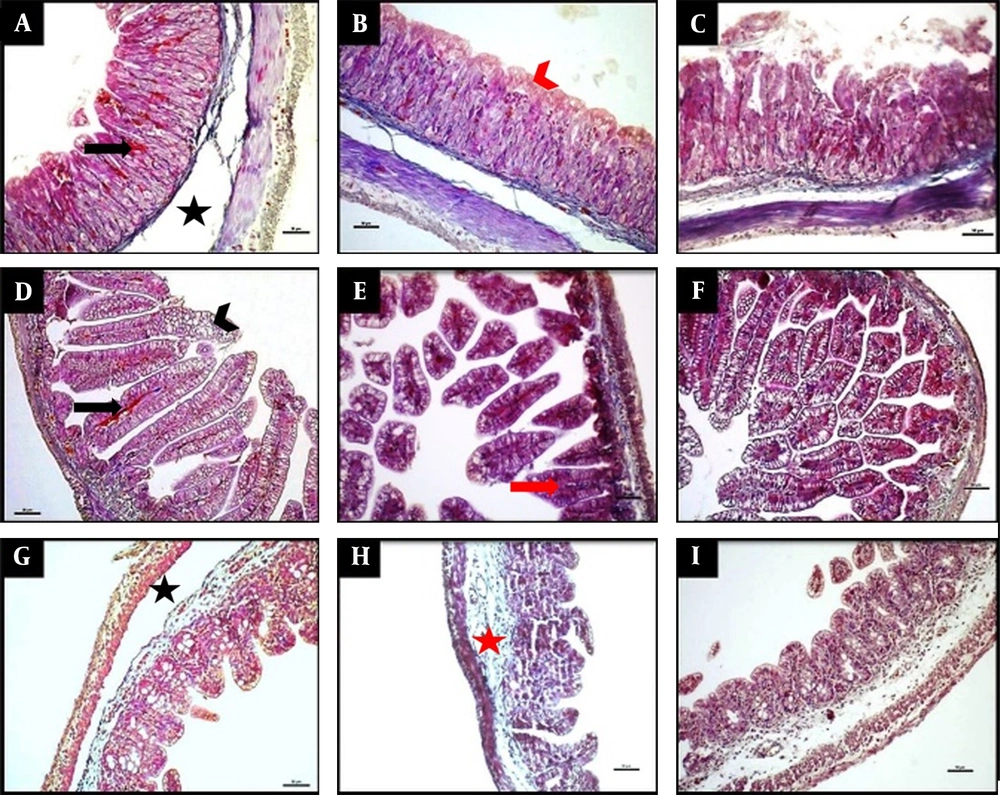
The results of histopathological injury scores are summarized in Table 1. The injury score was significantly higher in the NEC group than in the control and treatment groups at all GIS regions examined (P < 0.001; Figure 1). When the treatment group was compared with the control group, the injury score in the treatment group was significantly higher in the stomach and ileum (P < 0.01 and P < 0.001, respectively).
Abbreviation: NEC, necrotizing enterocolitis.
a P < 0.001 compared with group I
b P < 0.001 compared with group III
c P < 0.01 compared with group I
d P < 0.001 compared with group I
Representative photomicrographs of histology of the stomach (A-C), ileum (D-F), and caecum (G-I) demonstrated by Masson trichrome staining. The NEC group (A, D, G), the NEC + flavonoid group (B, E, H), and the control group (C, F, I). The scale bar represents 50 µm. The NEC group (A, D, G) displayed separation of the submucosa (black asterisk), congestion (black arrows), and villous destruction (arrowhead). The NEC + flavonoid group (B, H, E) showed intact epithelium, red arrowhead), shortened villi (red arrow), and submucosal edema (red asterisk). The control group (C, F, I) revealed normal histological findings.
4.2. Biochemical Findings
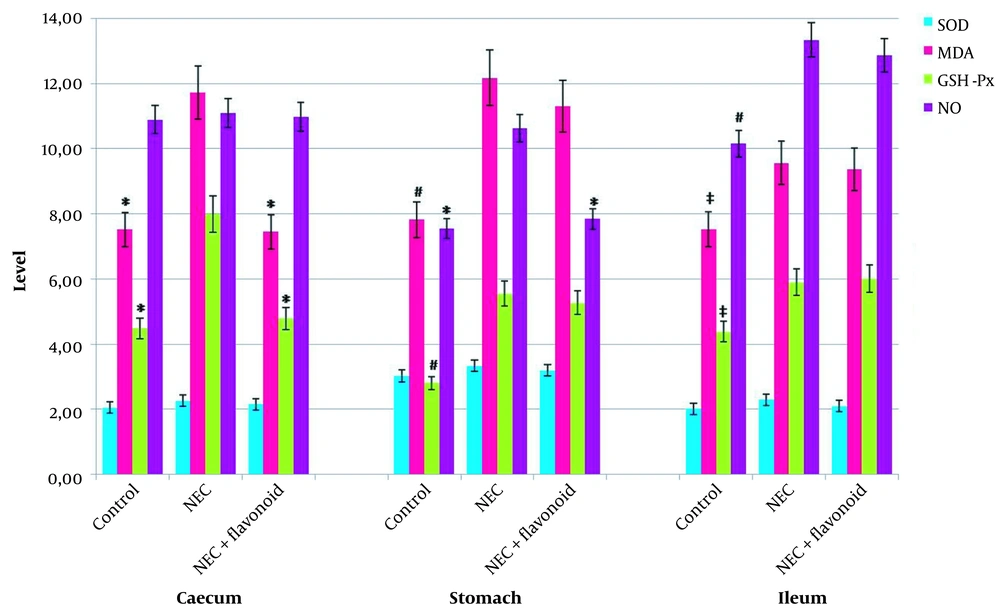
SOD, MDA, GSH-Px, and NO levels are presented in Figure 2. GSH-Px activity and MDA levels were significantly higher in the NEC group than in the control group in all examined GIS regions (P < 0.01 for the ileum and P < 0.001 for the caecum and stomach). When we compared the NEC group with the treatment group, it was seen that in the NEC group, the cecum levels of GSH-Px and MDA were significantly higher compared to the control group. Similarly, NO levels in the stomach were also substantially elevated in the NEC group (P < 0.001). GSH-Px and MDA levels were significantly higher in the stomach and ileum, whereas NO levels were significantly higher only in the ileum in the treatment group compared to the control group (P < 0.001 for GSH-Px and MDA levels in the stomach and NO levels in the ileum. P < 0.01 for GSH-Px and MDA levels in the ileum).
4.3. Apoptotic Index Results
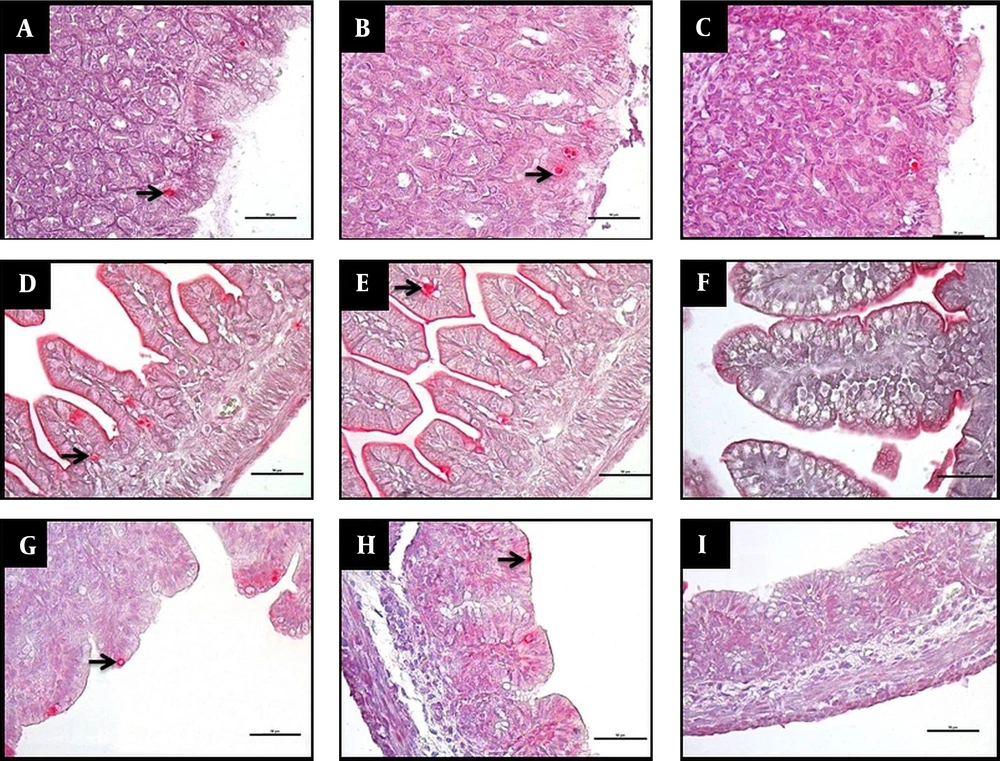
The apoptotic index findings are shown in Table 2. The apoptotic index was significantly higher in the NEC group than in the control and treatment groups in all examined GIS regions (P < 0.001; Figure 3). Regarding the ileum, apoptotic index values were significantly higher in the treatment group than in the control group (P < 0.01).
Abbreviation: NEC, necrotizing enterocolitis.
a P < 0.001compared with group I
b P < 0.001 compared with group III
c P < 0.01 compared with group I
5. Discussion
The effect of hypoxia/reoxygenation injury is crucial in the development of NEC (26-28). It was seen that the administration of Vitamin E, an antioxidant, decreased histopathological damage and lowered MDA levels in rats with hypoxia-induced NEC (28). Guven et al. (29) compared the animals with an induced NEC model with the NEC + ozone treatment group. The mortality rate, weight loss, and histopathological injury score were significantly lower in the NEC + ozone group than in the NEC group. However, oxidative stress markers (MDA and protein carbonyl content [PCC]), nitrite + nitrate levels, and TNF-α levels were low, and antioxidant enzyme activities (SOD and GSH-Px) were higher in the NEC + ozone group than in the NEC group (29). It has been shown that caffeic acid phenyl ester (CAPE), an antioxidant, reduces inflammation and apoptosis in the intestines of NEC-induced rats while decreasing the degenerative score and total oxidant level (30). It has been reported that the dexpanthenol (19) and melatonin (31) show similar effects on NEC-induced rats. When investigating the protective effect of colchicine, an anti-inflammatory agent against NEC, MDA, TNF-α, and IL-1β levels were lower, and SOD and GSH-Px levels were higher in the NEC + colchicine group than in the NEC group. The intestinal injury score was also significantly high in the NEC group (16). Intraperitoneal administration of N-acetyl cysteine (NAC), a free radical scavenger and antioxidant precursor, significantly reduced intestinal mucosal damage in NEC. However, a decrease in MDA, an increase in SOD, and a decrease in intestinal TNF-α were found in the NEC + NAC group than in the NEC group (17). It was demonstrated biochemically that experimental administration of enteral surfactant yields protection against NEC in rats (20). It has been found that intestinal alkaline phosphatase (IAP) administration decreases the degree of intestinal damage in NEC in a dose-dependent manner. It has also been reported that increased intestinal permeability in NEC is improved by IAP administration (18). In a genetic study conducted in human cell cultures, it has been found that probiotics have protective properties in NEC (32). We notice that antioxidants are used more in NEC-related studies. In our study, by using a molecule that is clinically used as an antioxidant, antiapoptotic, anti-inflammatory, and microcirculatory agent, we attempted to examine the effect of this molecule, which is thought to have some impacts on NEC pathophysiology.
In our literature review, we found several studies investigating the antioxidant, antiapoptotic, and anti-inflammatory effects of Hsd and diosmin. Antioxidant activity was assessed by intravenous administration of Hsd (100 mg/kg) per day in infertility associated with ovarian toxicity induced by cyclophosphamide (CP) in rats. In the CP + Hsd treatment group, NO and MDA levels and Myeloperxidase activity decreased, and SOD and GSH-Px activities increased compared to the CP group (33). In cell culture studies, it has been shown that administration of diosmin in lipopolysaccharide (LPS)-induced apoptosis increases the proapoptotic Bad protein expression, increases antiapoptotic Bcl-2 protein expression, and enhances antiapoptotic effects by reducing LPS-induced caspase-3 activation (34).
The drugs used in this study included 90% diosmin and 10% Hsd flavonoids. Although in several studies, it has been found that this medication is effective in reperfusion injury, the mechanism of this effect has not been fully elucidated.
In our study, it was found that flavonoid administration in rats with NEC reduced the apoptotic index and histopathologic injury score in the whole gastrointestinal system and biochemically decreased MDA in the cecum and NO in the stomach in accordance with the findings in the literature. However, contrary to the literature, our study showed that flavonoid administration reduced GSH-Px levels in the cecum. We believe that further comprehensive studies may be useful in this regard.
5.1. Conclusions
Since there is no routine parameter for the efficacy of NEC damage and treatment, our study looked at lipid peroxidation products and antioxidant levels to establish the efficacy of Hsd + diosmin on NEC. We think that these parameters provide valuable information. However, inflammatory mediators (PAF, TNF-α, NO, and IL-6) were not measured in our study, which is a limitation of this study. We also could not evaluate the effects of flavonoids on microcirculation in this study. We think that a comprehensive study that includes mediators involved in NEC and microcirculation would more reliably establish the impact of flavonoids on NEC.
5.2. Limitations
Small number of animals.